Antibody Conjugation
Antibody Conjugation Detection Methods
Antibodies conjugated with fluorophores, biomolecules, or enzymes are essential to both direct and indirect detection methods in immunoassays. Every immunoassay-based experiment requires antibodies with specific types of labels/conjugates: for example, flow cytometry uses fluorescently labeled antibodies, while horseradish peroxidase (HRP)-labeled antibodies are useful for chemiluminescence-based detection in western blot analysis.
In immunoassays, there are two common detection methods used to identify the antigen of interest: direct detection, and indirect detection. The method of detection will determine whether a conjugated primary antibody or secondary antibody is required.
Direct Detection Method
One-step immunolabeling procedure in which a conjugated primary antibody binds its target antigen or analyte generating a detection signal in the sample being tested.
Pros
Low background signal, minimal species cross-reactivity, great for multiplexing
Cons
Lower sensitivity, less flexibility due to limited availability of conjugated primary antibodies
Indirect Detection Method
Two-step method in which an unconjugated primary antibody binds the target antigen or analyte, and then a conjugated secondary antibody detects the bound primary generating the final signal.
Pros
More flexible, highly sensitive as the signal is amplified by secondary antibody
Cons
Limited number of primary antibody host species limits multiplexing
Fluorescent Detection for Immunoassays
Fluorophores – also called fluorescent probes, fluorescent dyes, or fluorochromes – are proteins, organic small molecules, or synthetic polymers capable of absorbing light at a specific wavelength and then emitting the light at much longer wavelength(s). They have become essential to mainstream immunoassay techniques in modern science, especially in microscopy and flow cytometry.
One of the key benefits of using fluorophore-based immunoassays is that they enable the detection of antigens expressed in very low abundance. Additionally, each fluorophore exhibits unique spectral properties, allowing for simultaneous detection of multiple signals, e.g. fluorophores enable multiplexing options in immunoassays.
Advantages of the DyLight® Fluorophores
The DyLight® fluorophores exhibit fluorescence emission covering nearly the entire visible light spectrum. DyLight® dyes exhibit higher or comparable fluorescence intensities and greater photostability compared to their analogous Alexa Fluor®, CyDye™, and Li-COR® dyes. Wide Stokes shifts, narrow absorption/emission spectra, and an excellent aqueous solubility are a few other highlights of DyLight® fluorophores.
Importantly, antibodies labeled with DyLight® have good aqueous solubility and stability over a broad pH (pH 4.0–9.0) which makes them ideal for multiplexing in cell-free as well as cell-based immunoassays.
Applications of DyLight® Conjugated Antibodies
See examples of our DyLight®-conjugated antibodies in action:
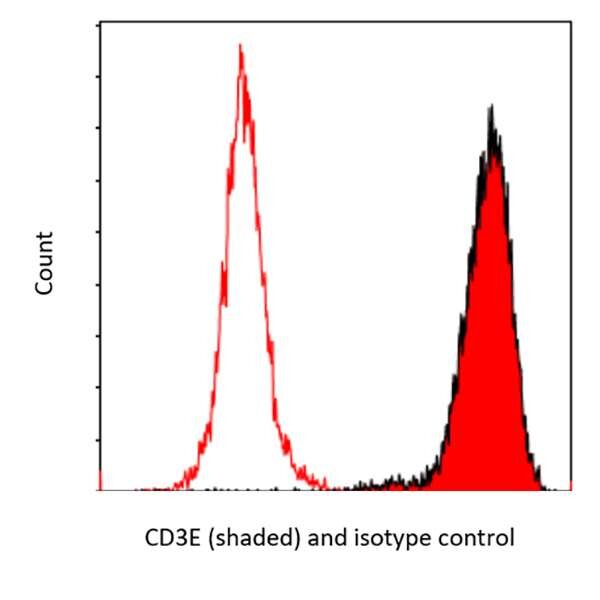
Flow Cytometry. Detection of CD3E by flow cytometry using anti-CD3E recombinant mAb (shaded red) [clone BL-298-5D12] [A700-016] and a DyLight® conjugated Goat-anti Rabbit IgG secondary antibody [A120-201D5].
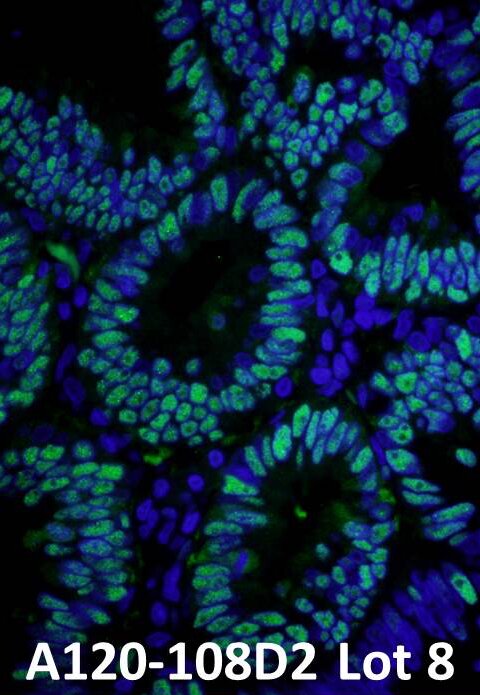
IHC. Detection of PCNA by immunofluorescence using anti-PCNA (green) [IHC-00012] and a DyLight® conjugated Donkey-anti Rabbit IgG secondary antibody [A120-108D2].
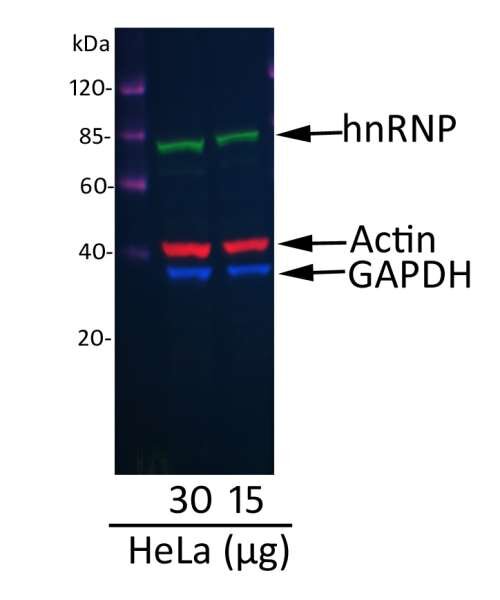
Western blot. A fluorescent western blot of hnRNP showing the multiplex use of our DyLight® 680 Conjugated Donkey anti-Mouse IgG-Heavy and Light Chain Cross-Adsorbed secondary antibody (red) [A90-337D6], with two other fluorescent labeled antibodies.
Spectral Characteristics of DyLight® and Other Fluorophore Options
| Fluorescent Conjugate | Excitation Wavelength (nm) | Emission Wavelength (nm) | Emission Color | Spectrally Equivalent Dyes |
|---|---|---|---|---|
|
Cy2® |
483-485 |
500 |
Green |
Alexa Fluor® 488, ATTO® 488, CF® 488A, DyLight® 488, FITC, Fluorescein |
|
Cy3® |
554 |
566 |
Yellow |
Alexa Fluor® 555, CF®555, DyLight® 550, TRITC |
|
Cy5® |
649 |
666 |
Red |
Alexa Fluor® 647, ATTO® 647N, CF®640R, CF®647, DyLight® 650 |
|
Cy5.5® |
672-673 |
690 |
Red |
Alexa Fluor® 680, CF®680, DyLight® 680, IRDye® 680 |
|
DyLight® 350 |
351 |
434 |
Blue |
Alexa Fluor® 350, AMCA, CF®350 |
|
DyLight® 488 |
492 |
519 |
Green |
Alexa Fluor® 488, ATTO® 488, CF®488A, Cy2®, FITC, Fluorescein |
|
DyLight® 550 |
553 |
569 |
Yellow |
Alexa Fluor® 555, CF®555, Cy3®, TRITC |
|
DyLight® 594 |
591 |
617 |
Orange |
Alexa Fluor® 594, ATTO® 594, CF®594, Texas Red |
|
DyLight® 650 |
651 |
673 |
Red |
Alexa Fluor® 647, ATTO® 647N, CF®640R, CF®647, Cy5® |
|
DyLight® 680 |
676 |
705 |
Red |
Alexa Fluor® 680, CF®680, Cy5.5®, IRDye® 680 |
|
DyLight® 755 |
750 |
771 |
Near Infrared |
Alexa Fluor® 750, CF®750, Cy7®, APC, IRDye® 750 |
|
DyLight® 800 |
770 |
798 |
Infrared |
CF®770, IRDye® 800 |
|
FITC |
495 |
519 |
Green |
Alexa Fluor® 488, ATTO® 488, CF®488A, DyLight® 488, FITC, Fluorescein |
|
PE |
565 |
576 |
Red |
Alexa Fluor® 568, ATTO® 565, CF®568 |
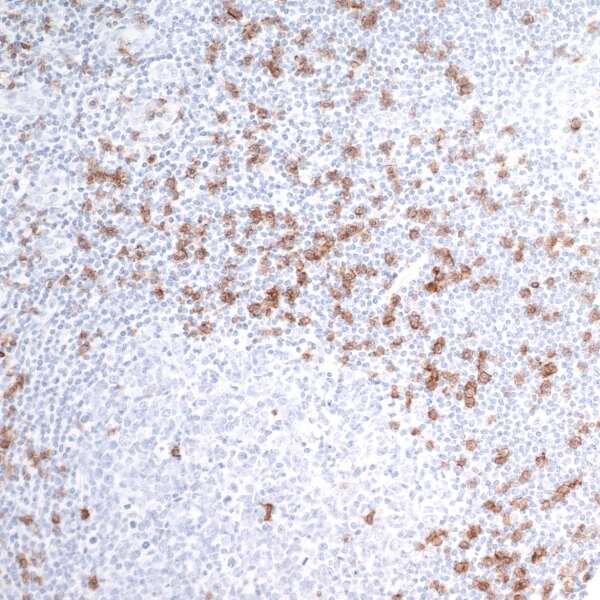
IHC of human tonsil with rabbit anti-CD8 alpha recombinant mAb [clone BLR044F] [A700-044] and HRP conjugated anti-rabbit secondary antibody followed by DAB-based detection.
Enzyme Conjugated Antibodies
Horseradish peroxidase (HRP) and alkaline phosphatase (ALP) are the two most common enzymes conjugated to antibodies for immunodetection applications. HRP and ALP act on chromogenic substrates to catalyze chemical reactions which produce insoluble, visible precipitates.
The choice between HRP and ALP often depends on the specific requirements of the assay and the nature of the sample being tested. HRP is known for its high sensitivity and rapid reaction time, making it ideal for detecting low-abundance antigens. On the other hand, ALP is favorable in situations where a longer reaction time is beneficial, as it can produce a more stable signal over extended periods.
The quantity of chromogen produced from these reactions can serve as an indicator of the amount of antigen detected in the immunoassay. Additionally, chromogen deposition in IHC/ICC can provide spatial information to determine localization of antigens within the tissue or cells being analyzed. The benefits of chromogenic detection include stability of signal, low cost, and integration into many optical detection systems such as light microscopy and spectrophotometry.
Chromogenic detection with enzyme labeled antibodies is useful for ELISA, IHC/ICC, and western blot immunoassays.
Signal Amplifying Conjugates
In several types of immunoassays, researchers can facilitate the detection of low abundance target antigens by using antibodies conjugated to signal amplifying molecules. Biotin conjugated antibodies are commonly used for signal amplification in immunoassays.
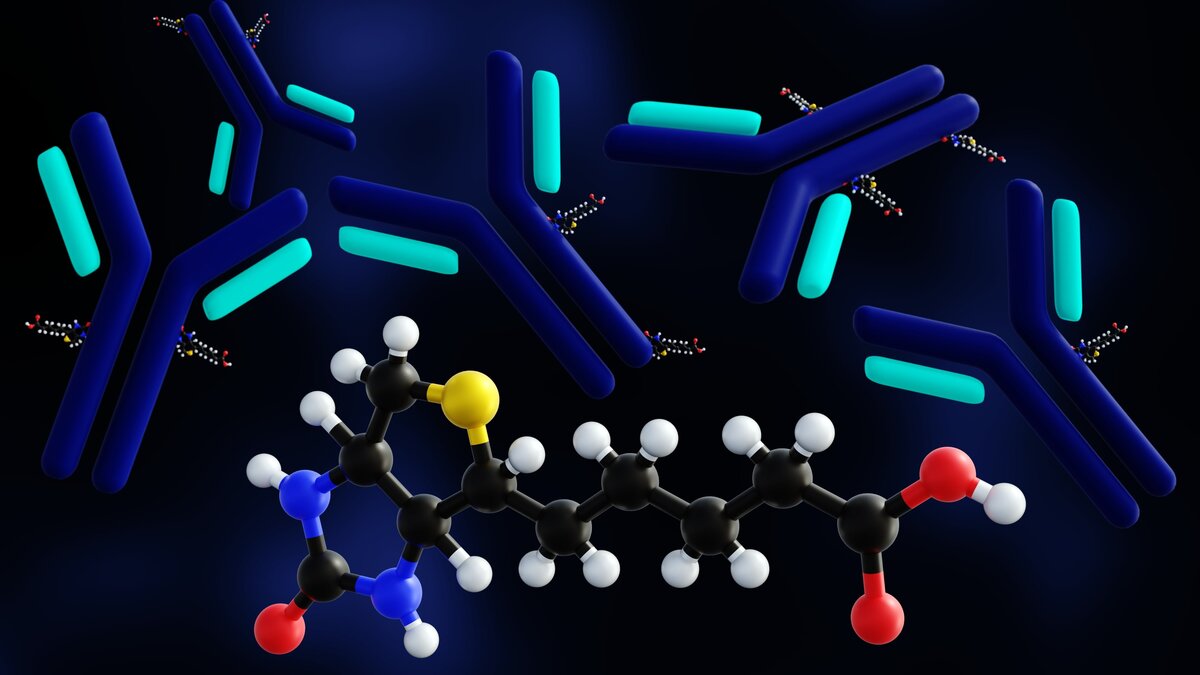
Multiple biotin molecules can bind to an antibody during the conjugation process. During the detection stage of an immunoassay, four molecules of streptavidin bind to each of the biotin molecules on the antibody. The streptavidin molecule may itself be conjugated to a fluorophore or other molecule to facilitate visibility of the complex. Accordingly, the biotin-streptavidin combination significantly amplifies the detection signal. This amplification is crucial, especially when dealing with low concentrations of target antigen, as it enhances the sensitivity of the assay. By increasing the abundance of detectable signals, researchers can more accurately identify the presence of specific proteins or other biomolecules in a sample.
In addition to biotin-streptavidin systems, other signal amplifying conjugates are also employed in immunoassays. For instance, enzyme-linked antibodies can catalyze reactions that produce a detectable signal, such as colorimetric changes or luminescence. Common enzymes used in these systems include horseradish peroxidase (HRP) and alkaline phosphatase (AP). When substrates are added to the reaction, these enzymes convert them into insoluble dye products that can be easily visualized, further enhancing the assay's sensitivity.
DyLight® is a registered trademark of Thermo Fisher Scientific Inc. and its subsidiaries. We are licensed to offer DyLight® antibody conjugates which we make at our antibody facility and validate in our in-house QC lab.
Alexa Fluor® is a registered trademark of Life Technologies Corp.
Cy and CyDye are trademarks of Cytiva.
LI-COR is a trademark of LI-COR, Inc.