ELISA
ELISA, which stands for Enzyme-Linked Immunosorbent Assay, is an immunological assay commonly used to measure the presence and concentration of proteins or other analytes within a biological sample. Like other types of immunoassays, ELISA relies on antibodies to detect the target antigen based on antibody-antigen interactions.

What Is ELISA Used For?
ELISA is a robust technique that allows scientists to both detect the presence of a specific protein in samples and quantify the amount of protein present. ELISA is quick and easy to perform and is a high-throughput immunoassay.
Samples may include:
- Serum, plasma, milk, or other biological fluids
- Cell culture supernatant
- Tissue homogenates
ELISA is one of the most sensitive immunoassays available. Experimentally, this means that ELISA can be used to compare differences in protein concentration between control and experimental groups in an in vivo or in vitro experiment, or between healthy donor and patient samples in clinical studies.
How Does ELISA Work?
To perform an ELISA assay, the target antigen must be immobilized, either directly through adsorption to the assay plate or indirectly via an antibody that is attached to the plate.
There are many types of ELISA tests for matching antibodies, which are categorized based on how analytes and antibodies are bound. Each ELISA format offers unique advantages and is selected based on factors such as the nature of the antigen, desired sensitivity, specificity requirements of the assay, and availability of matching antibody pairs. These are reviewed below.
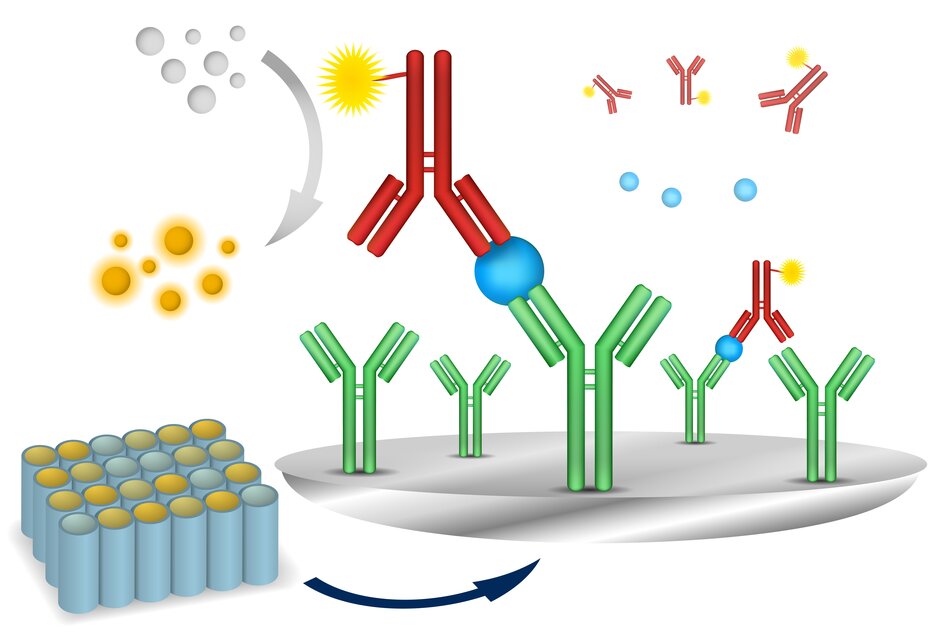
Bethyl ELISA kits are based on a sandwich ELISA. The antigen present in the test sample is captured by an antibody that has been pre-adsorbed on the surface of microtiter wells. After sample binding, unbound proteins and molecules are washed off and a biotinylated detection antibody is added to the wells to bind the captured antigen.
No matter the method used to immobilize the antigen to the plate, the enzymatic reaction used to detect and quantify the amount of analyte present in the sample is the same. Quantification of the target antigen is based on an enzymatic reaction. Upon addition of the enzyme substrate, the enzymatic reaction produces a measurable signal, typically a color change, which is directly proportional to the amount of bound antigen. A streptavidin-conjugated horseradish peroxidase (SA-HRP) is then added to catalyze a colorimetric reaction with the chromogenic substrate TMB (3,3’,5,5’-tetramethylbenzidine). The colorimetric reaction produces a blue product, which turns yellow when the reaction is terminated by the addition of dilute sulfuric acid.
The absorbance of the yellow product at 450 nm is proportional to the amount of analyte present in the sample and a four-parameter standard curve can be generated. The analyte concentrations in the test samples can then be quantified by interpolating their absorbance from the standard curve generated in parallel with the samples. After factoring sample dilutions, the antigen concentrations in the original sample can finally be calculated.
(A variant of this technique, known as FLISA for Fluorescence-Linked Immunosorbent Assay, employs a fluorophore-conjugated antibody to generate a fluorescent signal upon excitation by an appropriate light source.)
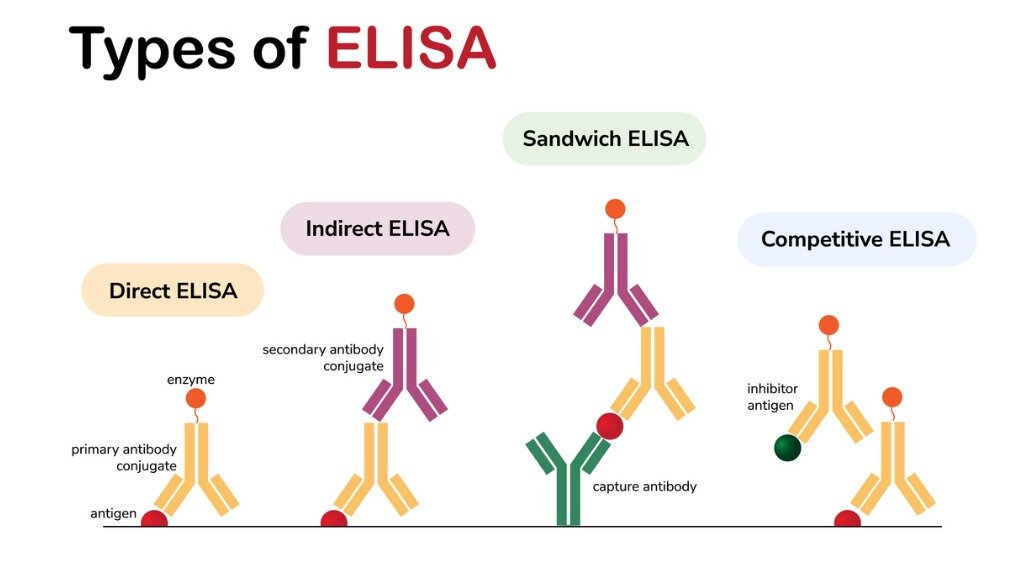
Direct ELISA Method
In the direct ELISA method, the antigen is directly immobilized on the surface of the plate and then detected with a specific antibody for the target antigen. This primary antibody is directly conjugated to an enzyme, such as HRP, that is used for detection.

Indirect ELISA Method
In the indirect ELISA method, the primary antibody binds to the antigen that is immobilized to the surface of the plate, before a secondary antibody binds recognizing the primary antibody is added for detection. Indirect and direct ELISA are similar in that the antigen is directly bound to the plate; direct ELISA is a single-step protocol while indirect ELISA requires two steps.
Sandwich ELISA Method
The sandwich ELISA method is different from the direct and indirect ELISA methods in that the antibody is immobilized to the surface of the plate by a capture antibody, which is coated onto the plate before the sample is added. If any antigen is present in the sample, the antigen binds to the capture antibody when it is added to the wells. A detection antibody is then added, binding to the antigen, before an enzyme-linked secondary antibody is added that binds the detection antibody. Bethyl ELISA kits are based on the sandwich ELISA methodology.
Protocols for ELISA
ELISA Resources
Whitepaper: Eight tips for ensuring a successful ELISA
Article: Albumin is a useful marker of organ function and tissue damage
Article: Defeating COVID-19: The science behind a new ELISA for COVID-19 seroconversion detection
eBook: A Comprehensive Guide to ELISAs
Whitepaper: Bovine Serum Albumin (BSA) Quantification with ELISA
ELISA Reagents
ELISA FAQs
Can I use other ELISA buffers (PBS) and substrates (ABTS or OPD) with Fortis Life Sciences ELISA kits?
Do Fortis ELISA kits contain polyclonal or monoclonal antibodies?
What type of samples can I use with Fortis ELISA kits?
How should I store my samples before use?
How do I dilute my samples?
What if my raw OD values are too low or too high?
What can I do to decrease the background signal?
The quantification of my sample is not consistent or seems to be incorrect. What can I do to correct this problem?
Where can I buy the stop solution?
What type of software is needed to graph a 4-parameter curve?
I do not have software that will perform a 4-parameter standard curve. What should I use to analyze my data?