Clinically used broad-spectrum antibiotics compromise inflammatory monocyte-dependent antibacterial defense in the lung
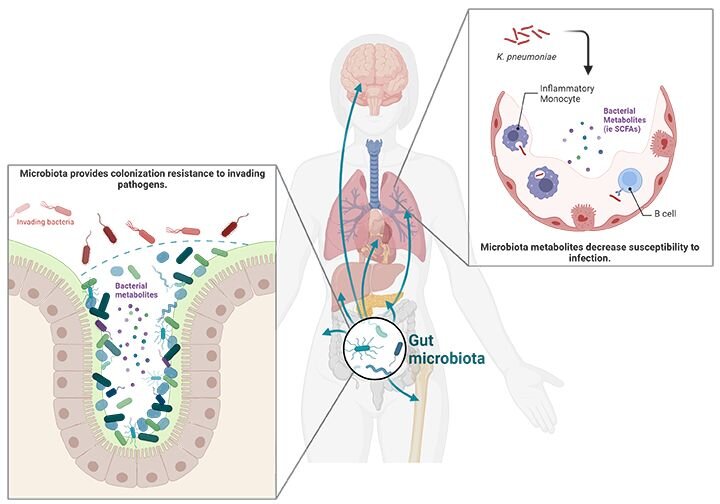
Hospital-acquired pneumonia (HAP) is the most common cause of hospital-acquired infections in the US and Europe. Antibiotic use is a significant risk factor for HAP but the mechanisms involved in this risk factor remain poorly understood. This recent study explored microbiome diversity and the impact of transplanting microbiota samples from antibiotic treated and antibiotic naive patients into wild type mice or those lacking short chain fatty acid (SCFA) receptors. These experiments highlighted the role of the naïve microbiota for inducing SCFAs that play a critical role in antimicrobial activity of inflammatory monocytes in the lung.
Hospital Acquired Pneumonia
Hospital-acquired pneumonia (HAP) is defined as pneumonia that occurs 48 hours or more after hospital admission and is not present at the time of admission. Occurring at a rate of 5-10 per 1000 hospital admissions, HAP is the most common cause of hospital-acquired infections in the US and Europe.1 It is also the number one cause of death from hospital-acquired infections.2 Common pathogens of HAP include Psuedomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, and Staphylococcus aureus, many of which have strains that are multidrug-resistant. The most common risk factor for HAP is previous use of antibiotics,1 specifically against anerobic bacteria.2
Benefits of the Microbiota
Antimicrobial therapy disrupts the microbiota and reduces many of its important beneficial and protective effects. One common impact of antimicrobial therapy is the disruption of colonization resistance, which is the ability of the microbiota to protect its host from the colonization of other, potentially harmful microbes. This was first observed when antibiotic treatments increased the risk associated with gastrointestinal infections like Clostridium difficile.3 Additionally, the microbiota primes immune defense pathways not only in the gut but throughout the body to enable processes like protection against pulmonary infections.2
Antimicrobial treatments reduce short-chain fatty acid levels
Currently, there is an incomplete understanding of how antimicrobial treatments affect susceptibility to HAP and no targeted prophylactic interventions exist. This inspired researchers in a recent study to investigate the gut microbiota of hospitalized patients and use mouse transplant models to assess impacts on immune defense against multidrug resistant K. pneumoniae.2 Fecal samples were collected from 72 hospitalized patients, 36 receiving broad spectrum antibiotics and 36 not receiving antibiotics. Microbiome analysis of a subgroup of these patients indicated no difference in total bacterial loads between the groups. However, shotgun metagenomic sequencing on 55 patient stool samples (26 antibiotic, 29 no antibiotic) showed decreased microbial diversity in the samples from antibiotic-treated patients characterized by lower amounts of commensal bacteria of the order Clostridiales.2
Many Clostridiales bacteria are known to produce short-chain fatty acids (SCFAs). Functional profiling showed that the antibiotic-treated samples had decreased functional modules linked to SCFA production and this correlated with decreased SCFA plasma levels.2 SCFAs are microbe waste products that mediate a variety of effects on the host organism. They can be used for energy production, gene regulation, and cell differentiation. SCFAs can act locally or be absorbed and systemically circulated, so they can impact cells throughout the host.3
Microbiota transplant model replicated patient findings
To investigate the role of broad-spectrum antibiotics on antibacterial defenses and the possible involvement of SCFAs, wild type (WT) and Ffar2/Ffar3-/- mice lacking SCFA receptors FFAR2 and FFAR3 were treated with an antibiotic cocktail to deplete their normal microbiota and then transplanted with fecal material from the hospitalized patients either receiving antibiotics or not (antibiotic-associated microbiota and antibiotic-naïve microbiota, respectively). Twenty-six different patient stool samples were transplanted into 46 animals so that in most cases pairs of WT and Ffar2/Ffar3-/- mice received an individual patient’s microbiota. This model was able to replicate the findings observed in the patient microbiome analysis that antibiotic treatment decreased microbial diversity in the gut and had lower amounts of Clostridiales bacteria.
The microbiota-transplanted mice were then intranasally infected with multidrug-resistant K. pneumoniae. WT antibiotic-naïve microbiota mice had decreased bacterial loads compared to WT antibiotic-associated microbiota mice after 24 hours. The antibiotic-associated mice also had enhanced neutrophilic inflammation and lung barrier damage demonstrated by increased myeloperoxidase levels and serum albumin leakage into bronchoalveolar lavage fluids measured with the Mouse Albumin ELISA Kit (E99-134) from Bethyl Laboratories, Inc.
SCFAs enhance antimicrobial activity of inflammatory monocytes
To determine the mechanism by which SCFAs affect bacterial immunity, immune cells and cytokine levels were examined at 12 hours post inoculation. No differences were observed in inflammatory cytokine production (IL-1β, TNFα, CXCL1, CXCL2, CXCL5, CCL2) or recruitment of immune cells classically associated with antibacterial defenses (alveolar macrophages, polymorphonuclear neutrophils (PMNs), inflammatory monocytes (IM), dendritic cells, natural killer cells). The researchers then investigated activation status and gene expression in immune cells using single-cell RNA sequencing. Data from these studies suggest that SCFAs affect antibacterial activity of PMNs and inflammatory monocytes. Subsequently, alveolar macrophages, PMN, and inflammatory monocytes depletion experiments supported inflammatory monocytes as the key cell type involved in the protective effects of SCFAs. These results were further confirmed using adoptive transfer experiments.
Lastly, the effects of SCFAs on the microbicidal activity of inflammatory monocytes was examined by isolating them from the bone marrow of WT and Ffar2/Ffar3-/- mice and treating them with SCFAs prior to co-incubation with K. pneumoniae for two hours. SCFAs enhanced antimicrobial activity of the WT IMs but not Ffar2/Ffar3-/- IMs and these effects were blocked by the phagocytosis inhibitor cytochalasin D, the endolysosomal acidification blocker bafilomycin A1, and the lysozyme inhibitor N,N’,N"-Triacetylchitotriose. To test whether antibiotic-induced gut microbiota had similar effects on the antibacterial activity of inflammatory monocytes, cells were isolated from the bone marrow of mice transplanted with antibiotic-naïve or antibiotic-treated microbiota. The inflammatory monocytes from the mice transplanted with antibiotic-treated microbiota, and from the Ffar2/Ffar3-/- mice, irrespective of microbiota transplant, were unable to reduce K. pneumoniae levels. The data demonstrates that antibiotic-induced microbiota changes inhibit SCFA-controlled inflammatory monocyte-mediated microbicidal activity and that these effects depend on FFAR2/FFAR3, endosomal acidification, and lysozyme activity.2
This study provides a mechanism for the well-known connection between antimicrobial treatment and subsequent HAP. The researchers showed that broad-spectrum antibiotics deplete SFCA producing gut bacteria inhibiting the microbicidal activity of IMs. This work reinforces the need for prudent use of antibiotics and provides ideas for novel prophylactic strategies against HAP.
References
- Shebl E, Gulick PG. Nosocomial Pneumonia. In: StatPearls. StatPearls Publishing; 2023.
- Dörner PJ, Anandakumar H, Röwekamp I, et al. Clinically used broad-spectrum antibiotics compromise inflammatory monocyte-dependent antibacterial defense in the lung. Nat Commun. 2024;15(1):2788. doi:10.1038/s41467-024-47149-z
- Kuziel GA, Rakoff-Nahoum S. The gut microbiome. Current Biology. 2022;32(6):R257-R264. doi:10.1016/j.cub.2022.02.023