Serial Dilutions
A serial dilution is a step-wise and geometric series of dilutions which starts with a small amount of starting material and amplifies the dilution factor serially by using diluted material as a source for subsequent dilutions.
Serial two-fold and ten-fold dilutions are commonly used to titer antibodies or prepare diluted analytes (for a standard curve for example). Serial dilutions are also commonly used to avoid having to pipette very small volumes (1-10 µl) to make a dilution of a solution. Serial dilutions can be performed in tubes (microfuge or 12 X 75 mm) or in the wells of plates (depending on the volumes you require). It is important to know what volume of the dilution you will need and the range of concentrations that you desire.
In the example below we are performing two-fold serial dilutions of a \(1.0 \ mg \over ml\) stock of analyte for use as standards. We would like the concentration of the standards to be in the range of \(1000 \ ng \over ml\) to about \(10 \ ng \over ml\). The original or first dilution will dilute the stock for the highest primary concentration that we need, \(1000 \ ng \over ml\). This will be done by performing an initial 1: 1000 dilution because:
\[{C1 \over C2} = dilution factor\]
\[{1,000,000 \ ng/ml \over 1,000 \ ng/ml} = 1000 \ dilution factor\]
To prepare the initial 1:1,000 dilution we can take 5 µl of the stock solution and add 4,995 µl of diluent (buffer or water). This would result in a solution of analyte with a concentration of \(1000 \ ng \over ml\). Now the two-fold dilutions can be prepared from the \(1000 \ ng \over ml\) solution. For the first 1:2 dilution we would take 500 µl of the 1:1,000 dilution and add it to 500 µl of diluent in the next tube. This will result in a solution with a concentration of \(500 \ ng \over ml\). This solution is then used in a subsequent or serial dilution by taking 500 µl of the previous \(500 \ ng \over ml\) solution and adding it to the next tube containing 500 µl of diluent. This dilution will now result in a solution with a concentration of \(250 \ ng \over ml\). This process is repeated until a final solution at a concentration near \(10 \ ng \over ml\) is made. In the example below, the highest dilution has a concentration of \(7.8 \ ng \over ml\).
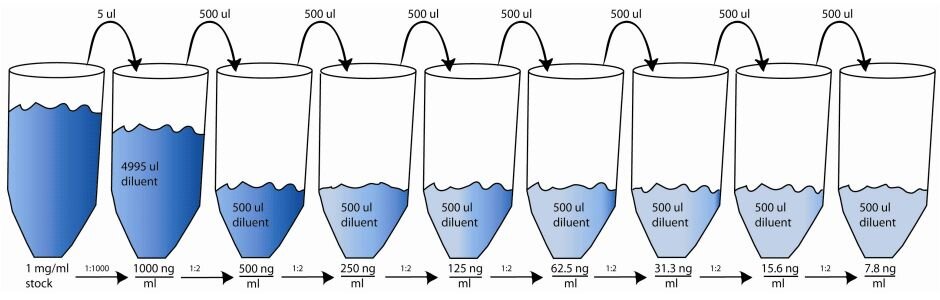
The total dilution factor for any of the resulting solutions can be calculated by multiplying each dilution factor leading up to a particular dilution. For example if we wanted to find the total dilution for the \(62.5 \ ng \over ml\) solution:
\[DFtotal = DF1 \times DF2 \times DF3 \times DF4 \times DF5\]
\[DFtotal = 1,000 \times 2 \times 2 \times 2 \times 2\]
\[DFtotal = 16,000\]
