USP11 is a potential new therapeutic target to inhibit autophagy in cancer
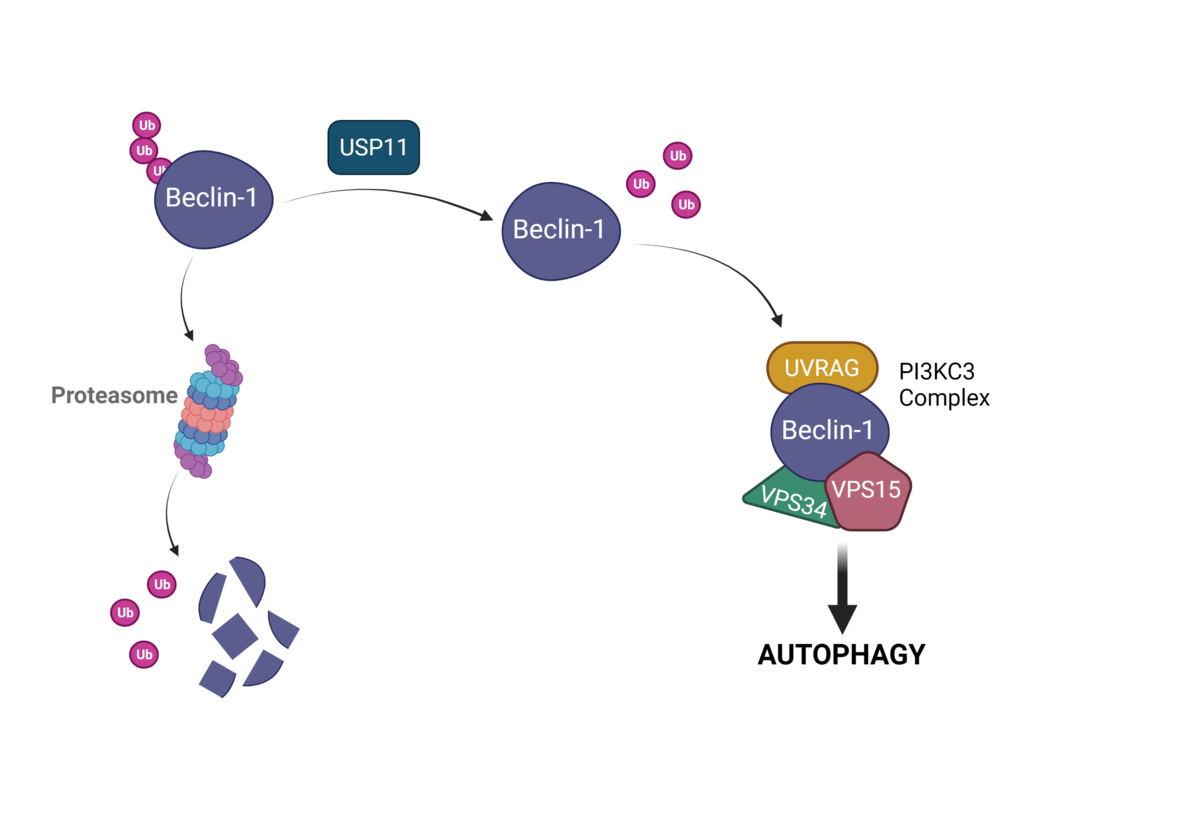
USP11 promotes autophagy by directly stabilizing Beclin-1. Autophagy is thought to promote tumor progression, and expression of USP11 and Beclin-1 both correlate with poor prognosis in cancer. A recent publication identifies USP11 as a potential therapeutic target to inhibit autophagy in cancer.
Autophagy is the process of using double membranes to sequester cytoplasmic contents for degradation and reuse. The initiation of these double membranes is still being heavily studied but is thought to originate from an autophagy protein accumulation site known as the pre-autophagosomal structure (PAS).1 Autophagy-related proteins, particularly ATG17 and Beclin-1 (B-cell lymphoma-2 interacting protein 1), are required for recruitment of other autophagy-related proteins at the PAS.2
Beclin-1 is a mammalian homolog of the yeast autophagy-related protein, ATG6. It forms a complex with vacuolar sorting protein-34 (VPS34) and vacuolar sorting protein-15 (VPS15) that generates phosphatidylinositol 3-phosphate (PtdIns-3P), the main component of the autophagosomal membrane. Additional autophagy proteins are recruited to this initial complex including UV irradiation resistance-associated gene (UVRAG) and activating molecule in Beclin-1 regulated autophagy (AMBRA-1), positive regulators of autophagy, along with RUN and cysteine rich domain containing Beclin-1 interacting protein (Rubicon), a negative regulator of autophagy.3
Beclin-1’s ability to interact with pro- and anti-autophagy factors is highly regulated through post-translational modifications. This protein has three acetylation sites, twenty-three phosphorylation sites, and nine ubiquitination sites, many of which can affect autophagy. Phosphorylation of Beclin-1 induces autophagy by increasing dissociation of Beclin-1 and BCL2 (an apoptosis-inducing factor), activating the production of PtdIns-3P, enhancing interactions with UVRAG, and more. However, phosphorylation of Beclin-1 also inhibits autophagy by enhancing the interaction between Beclin-1 and BCL2 and through enhanced interactions with vimentin intermediate filaments. Similar to phosphorylation, the ubiquitination sites can both promote autophagy by disrupting Beclin-1-BCL2 interactions, but can also lead to Beclin-1 degradation via the proteasome, reducing autophagy. Ubiquitin specific proteases (USPs) have been shown to play a role in de-ubiquitinating Beclin-1 increasing its stability, but the specific ubiquitination sites required for this destabilization have yet to be determined.3
USP11 (ubiquitin specific protease 11) had previously been found to interact with Beclin-1, VPS34, ATG10, ATG12, and ATG16L indicating a likely role in regulating autophagy. Multiple studies examined the role of USP11 in autophagy but failed to come to a consensus as to whether it promoted or inhibited autophagy. A recent study by Li et al. decided to tackle the discrepancies in the literature on the role of USP11 in autophagy by identifying the direct targets of the ubiquitin hydrolase.4
USP11 expression was reduced using shRNA and then rescued using shRNA-resistant wild type USP11 and a USP11 catalytically inactive mutant. The USP11 knockdown decreased autophagy as shown by elevated levels of P62 and decreased levels of LC3 II puncta. This phenotype was rescued with wild type USP11, but not the catalytically inactive mutant.
Additional studies showed that USP11 directly interacts with Beclin-1 and reduces Beclin-1 ubiquitin levels leading to increased Beclin-1 expression/stability. The increased Beclin-1 expression due to USP11 also increased the amount of VPS34, VPS15, ATG14, or UVRAG complex present in the cells. These observations required the catalytically active form of USP11. Taken together, Li et al. found that Beclin-1 is a substrate for the ubiquitin specific protease USP11, and that de-ubiquitination of Beclin-1 by USP11 promoted autophagy through Beclin-1 stabilization and PI3KC3 complex formation. This work provides an additional potential therapeutic target for diseases in which autophagy plays a major role.
Fortis Products Featured in the Article
| Name | Role in autophagy | Catalog # | Applications | Reactivity | Host | Clonality |
|---|---|---|---|---|---|---|
| USP11 | Promotes autophagy | A301-613A | IP, WB | Hu | Rabbit | Polyclonal |
| Beclin-1 | Autophagic protein localization to PAS | A302-566A | IP, WB | Hu, Ms | Rabbit | Polyclonal |
| A302-567A | IP | Hu | Rabbit | Polyclonal | ||
| VPS15 | Promotes autophagy | A302-571A | IP, WB | Hu, Ms | Rabbit | Polyclonal |
| P62/ Sequestrome-1 | Guides polyubiquitinated proteins & organelles to autophagosomes | A302-856A | IHC, IP, WB | Hu | Rabbit | Polyclonal |
References
- Mizushima N. Autophagy: process and function. Genes & Development. 2007;21(22):2861-2873. doi:10.1101/gad.1599207
- Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death & Differentiation. 2011;18(4):571-580. doi:10.1038/cdd.2010.191
- Menon MB, Dhamija S. Beclin 1 Phosphorylation – at the Center of Autophagy Regulation. Frontiers in Cell and Developmental Biology. 2018;6. doi:10.3389/fcell.2018.00137
- Li Z, Rao S, Song C, et al. Ubiquitin carboxyl-terminal hydrolase 11 promotes autophagy by de-ubiquitinating and stabilizing Beclin-1. Genome Instability & Disease. 2022;3(1):47-55. doi:10.1007/s42764-022-00061-6