Between Yes and No: B7-H4 and T cell Activation
Immunotherapy has become an increasingly important therapeutic option for treating both autoimmune diseases and various cancers. Based on the two-signal hypothesis for T-cell activation, the goal is to either decrease the deleterious effect of an overactive immune system as is the case with autoimmune diseases or the opposite, increase the immune response to enable targeted killing of cancer cells.
This is done by either activating or suppressing T cells via targeting immune checkpoint receptors and their respective ligands such as the CTLA-4 receptor and the ligand PD-L1. Although treatments targeting CTLA-4 and PD-L1 have had significant success in treating cancer a portion of patients do not respond.1,2,3 This has created a need for alternative immunotherapies targeting different B7 molecules like B7-H4 that may be effective in treating non-responding patients.
First proposed by Lafferty and Cunningham, the hypothesis that T cells require two-signals to sustain activation is the basis for many immunotherapies.4 The first signal needed for activation is the binding of MHC I/II molecules present on antigen presenting cells (APCs) to the T cell receptor5. While this activates key signaling pathways like PI3K-Akt-MTORC1, MAPK and NFkB, it is by itself not enough to maintain T cell activation.6,7,8 Therefore, a second signal coming from the activation of the T cell surface receptor CD28 by the B7 ligands CD80 and CD86 present on APCs is needed.9 During sustained activation, T cells begin to express immune checkpoint receptors like CTLA-4 that decreases the immune response by out competing CD28 for CD80 and CD86 depriving the T cell of its much needed second stimulus. Importantly, not all B7 molecules promote activation and can be suppressive. For example, the checkpoint receptor PD-1 when bound to the B7 ligand B7-H1 (PD-L1) blocks T cell proliferation, cytokine secretion and cytotoxic activity.7,8 Thus, the state of T cell activation is regulated between a balance of activating and suppressive signals from immune checkpoint receptors and B7 ligands.
Discovered in 2003, B7-H4 is a member of the B7 family of co-stimulatory proteins that includes B7.1 (CD80), B7.2 (CD86), B7-H1 (CD274, PD-L1), B7-H2 (ICOS-L), B7-H3 (CD276) and B7-DC (CD273, PD-L2) that suppresses T cell proliferation and cytokine production.10,11,12 Unlike other B7 proteins, B7-H4 mRNA is widely expressed in many tissues including the brain, heart, kidney, lung, ovary, pancreas and liver.10 But unlike its mRNA expression, there is very little to no protein expression of B7-H4 in normal tissues.13 Importantly, B7-H4 protein expression can be induced during inflammatory conditions with in vitro stimulation of cells with lipopolysaccharides, phytohemagglutinin and IFN-γ all increasing B7-H4 protein expression suggesting B7-H4 may be a valuable biomarker for the tumor microenvirnoment.10
Interest in B7-H4 as a target for treating cancer is born from the observation that it is overexpressed in both tumor cells and tumor-associated macrophages in a growing list of cancers including renal cell carcinoma.13, breast cancer14, ovarian cancer15,16 and pancreatic cancer17 Furthermore, the overexpression of B7-H4 is positively associated with disease progression including cancer stage, number T cells and patient survival18,19. Given that B7-H4 suppresses T cell activation, therapeutically blocking B7-H4 signaling is hypothesized to favorably alter the tumor microenvironment allowing of T-cell targeting of cancer cells.
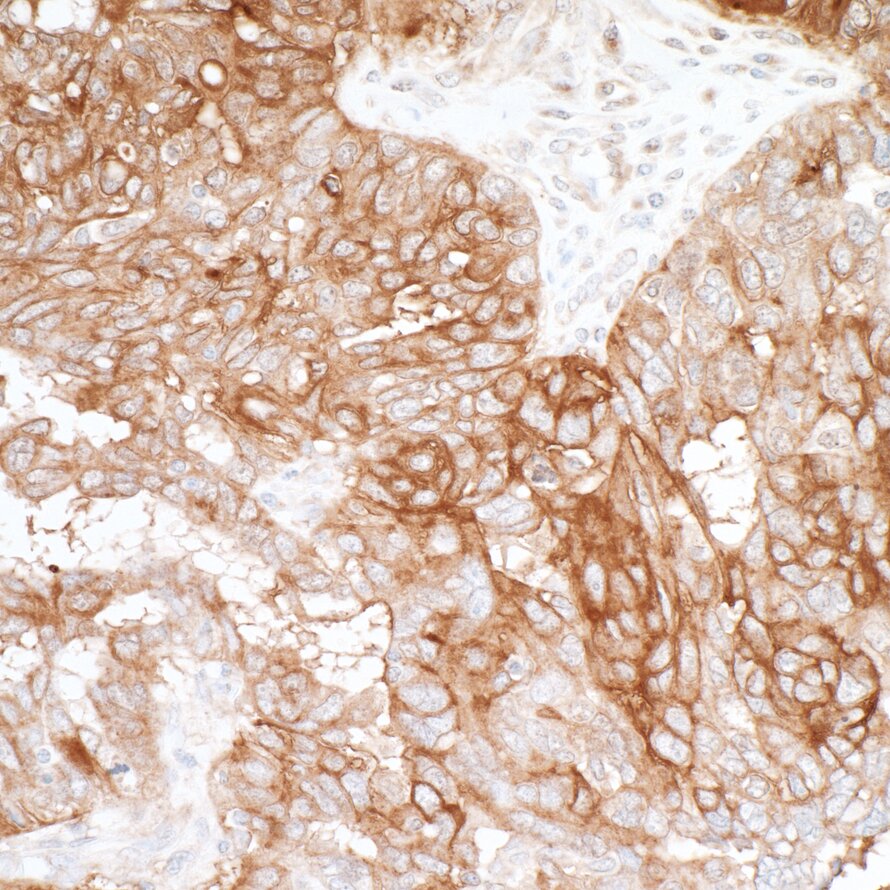
Detection of human B7-H4 by immunohistochemistry.
Taken together, while immunotherapy treatments targeting CTLA-4 and PD-L1 have been successful the fact that some patients that do not respond has created a need for new drug targets. Like PD-L1, B7-H4 is a suppressive B7 ligand that is thought to be hijacked by tumor cells to evade the immune response. Given B7-H4's ability to suppress T cell activation and its correlation for disease progression in a growing list of cancers B7-H4 may be a valuable treatment option for patients who do not respond to current immunotherapy treatments.
References
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. 2012. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 366(26):2455-2465.
- Ribas A, Camacho LH, Lopez-Berestein G, Pavlov D, Bulanhagui CA, Millham R, Comin-Anduix B, Reuben JM, Sega E, Parker CA, Sharma A, Glaspy JA, Gomez-Navarro J. 2005. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol. 23(35):8938-8977.
- Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, Herati RS, Mansfield KD, Patsch D, Amaravadi RK, Schuchter LM, Ishwaran H, Mick R, Pryma DA, Xu X, Feldman MD, Gangadhar TC, Hahn SM, Wherry EJ, Vonderheide RH, Minn AJ. 2015. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. 520(7547):373-377.
- Lafferty KJ, Cunningham AJ. 1975. A new analysis of allogeneic interactions. Aust J Ep Biol Med Sci. 53:27-42.
- Rotte A, Jin JY, Lemaire V. 2018. Mechanistic overview of immune checkpoints to support the rational design of their combinations in cancer immunotherapy. Ann Oncol. 29(1):71-83.
- Palucka K, Banchereau J. 2012. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 12(4):265-267.
- Combadiere B, Reis e Sousa C, Trageser C, Zheng LX, Kim CR, Lenardo MJ. 1998. Differential TCR signaling reglates apoptosis and immunopathology during antigen responses in vivo. 9(3):305-313.
- Guy CS, Vignali KM, Temirov J, Bettini ML, Overacre AE, Smeltzer M, Zhang H, Huppa JB, Tsai YH, Lobry C, Xie J, Dempsey PJ, Crawford HC, Aifantis I, Davis MM, Vignali DA. 2013. Distinct TCR signaling pathways drive proliferation and cytokine production in T cells. Nat Immunol. 14(3):262-270.
- Smith-Garvin JE, Koretzky GA, Jordan MS. 2009. T cell activation. Annu Rev Immunol. 27:591-619.
- Sica GJ, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, Chapoval AI, Flies DB, Bajorath J, Chen L. 2003. B7-H4, a Molecule of the B7 Family, Negatively Regulates T Cell Immunity. 18(6):849-861.
- Zang X, Loke P, Kim J, Murphy K, Waitz R, Allison JP. 2003. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci. 100(18):10388-10392.
- Prasad DV, Richards S, Mai XM, Dong C. 2003. B7S1, a novel B7 family member that negatively regulates T cell activation. 18(6):863-873.
- Salceda S, Tang T, Kmet M, Munteanu A, Ghosh M, Macina R, Liu W, Pilkington G, Papkoff J. 2005. The immunomodulatory protein B7-H4 is overexpressed in breast and ovarian cancers and promotes epithelial cell transformation. Exp Cell Res. 306(1):128-141.
- Krambeck AE, Thompson RH, Dong H, Lohse CM, Park ES, Kuntz SM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. 2006. B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci. 103(27):10391-10396.
- Tringler B, Zhuo S, Pilkington G, Torkko KC, Singh M, Lucia MS, Heinz DE, Papkoff J, Shroyer KR. 2005. B7-h4 is highly expressed in ductal and lobular breast cancer. Clin Cancer Res. 11(5):1842-1848.
- Krycek I, Zou I, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, Lackner A, Alvarez X, Ochoa A, Chen L, Zuo W. 2006. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 203(4):871-881.
- Simon I, Zhuo S, Corral L, Diamandis EP, Sarno MJ, Wolfert RL, Kim NW. 2006. B7-h4 is a novel membrane-bound protein and a candidate serum and tissue biomarker for ovarian cancer. Cancer Res. 66(3):1570-1575.
- Awadallah NS, Shroyer KR, Langer DA, Torkko KC, Chen YK, Bentz JS, Papkoff J, Liu W, Nash SR, Shah RJ. 2008. Detection of B7-h4 and p53 in pancreatic cancer: potential role as a cytological diagnostic adjunct. 36(2):200-206.
- Kryczek I, Wei S, Zhu G, Myers L, Mottram P, Cheng P, Chen L, Coukos G, Zou W. 2007. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res. 67(18):8900-8905.