CD68 and PD-L1: Better Together?
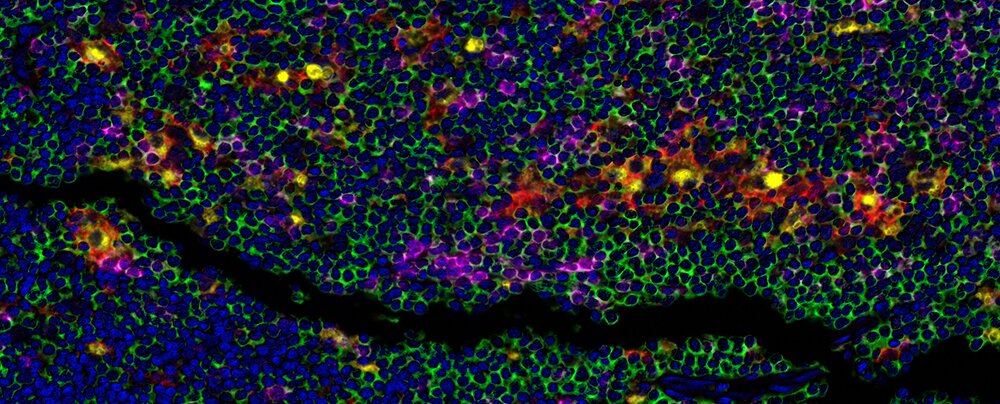
Detection of human CD3 (green), CD8 (magenta), CD68 (yellow), and PD-L1 (Red) in FFPE Hodgkin's lymphoma by mIF. Rabbit anti-CD3E recombinant monoclonal [BL-298-5D12] (A700-016), rabbit anti-CD8 alpha recombinant monoclonal [BLR044F] (A700-044), mouse anti-CD68 monoclonal [KP-1] (A500-018A), and rabbit anti-PD-L1 recombinant monoclonal [BLR020E] (A700-020). Secondary: HRP-conjugated goat anti-rabbit IgG (A120-501P) and HRP-conjugated goat anti-mouse IgG (A90-116P). Substrate: Opal™ 520, 570, 620, and 690. Counterstain: DAPI (blue).
Cluster of differentiation 68 (CD68) is a glycoprotein expressed primarily by macrophages that facilitates target identification by binding to tissue- or organ-specific lectins or selectins. CD68 is a macrophage panmarker, enabling identification of the entire macrophage population regardless of phenotype. M1 macrophages are proinflammatory and antitumoral, while the M2 phenotype is associated with protumoral and immunosuppressive effects1.
Further investigation of not only the colocalization of CD68 and PD-L1, but also any potential association between the colocalization of these two markers and clinical outcome is warranted to explore the potential for combination of anti-PD-1/PD-L1 drugs with drugs that target TAMs. Additional studies are also needed to further elucidate the role of CD68+ PD-L1+ cells specifically within the tumor microenvironment, as well as the impact of various anticancer treatments on these cells.
In this vein, multiple investigators have examined the use of an "immunuoscore" based on CD68 and PD-L1 to either predict the response to anticancer therapy or to select patients for therapy based on the likelihood of their response to treatment.7-8 To date, work on the association between CD68 and PD-L1 expression has been completed in breast cancer, non-small cell lung cancer, ovarian cancer, Hodgkin lymphoma, and bladder cancer.9-11 Continued work in this area should provide additional information regarding the significance of CD68 and PD-L1 colocalization, as well as potential associated implications for cancer treatment.
Tumor cells actively recruit M2 macrophages through paracrine loops or direct contact with membrane molecules. Programmed cell death protein 1 (PD-1) and its ligand (PD-L1) represent one pair of such membrane molecules, and they play an important role in recruitment of tumor-associated macrophages (TAMs).1
CD68 and PD-L1 are each integral to tumorigenesis and the response (or lack of response) to anticancer therapy. For example, increased CD68+ macrophage index is associated with metastasis, shorter disease-free interval, poor prognosis, and reduced overall survival in multiple types of cancer.1-4 Similarly, PD-L1+ TAMs have been identified at the interface between malignant and healthy tissue, and may create an immunoprotective barrier around the tumor, thereby increasing resistance to immunotherapy. In contrast, inhibition of PD-L1 downregulates M2 macrophages and subsequently decreases their protumoral effects.1
Given the distinct individual roles CD68 and PD-L1 play in tumor progression and response to immunotherapy, the exact distribution and co-localization of these two molecules may further affect these outcomes. Indeed, in patients with diffuse large B-cell lymphoma, up to 18.9% of tumor cells were CD68+, and 30-52% of these CD68+ cells were PL-L1+.5 This is in comparison to a median of 55% and 30% of CD68+ cells identified as PD-L1+ in patients with squamous cell carcinoma and adenocarcinoma, respectively.6 Thus, across cancer types, colocalization of CD68 and PD-L1 appears to be approximately 30-55%.
References
- Evrard D, Szturz P, Tijeras-Raballand A, et al. 2019. Macrophages in the microenvironment of head and neck cancer: potential targets for cancer therapy. Oral Oncol. Jan;88:29-38.
- Leek RD, Lewis CE, Whitehouse R, et al. 1996. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. Oct;56:4625-9.
- Mahmoud SM, Lee AH, Paish EC, et al. 2012. Tumour-infiltrating macrophages and clinical outcome in breast cancer. J Clin Pathol. Feb;65:159-63.
- Nabeshima A, Matsumoto Y, Fukushi J, et al. 2015. Tumour-associated macrophages correlate with poor prognosis in myxoid liposarcoma and promote cell motility and invasion via the HB-EGF-EGFR-PI3K/Akt pathways. Br J Cancer. Feb;112:547-55.
- McCord R, Bolen CR, Koeppen H, et al. 2019. PD-L1 and tumor-associated macrophages in de novo DLBCL. Blood Adv. Feb;3(4):531-40.
- Sepesi B, Federico L, Mitchell K, et al. 2018. PD-L1 expression is predominant in CD68+ tumor-associated macrophages in stage I-III non-small cell lung cancers.
- Silva MA, Ryall KA, Wilm C, et al. 2018. PD-L1 immunostaining scoring for non-small cell lung cancer based on immunosurveillance parameters. PLoS One. Jun;13(6):e0196464.
- McLemore LE, Janakiram M, Albanese J, et al. 2018. An immunoscore using PD-L1, CD68, and tumor-infiltrating lymphocytes (TILs) to predict response to neoadjuvant chemotherapy in invasive breast cancer. Appl Immunohistochem Mol Morphol. Oct;26(9):611-9.
- Qu QX, Huang Q, Shen Y, et al. 2016. The increase of circulating PD-L1-expressing CD68(+) macrophage in ovarian cancer. Tumour Biol. Apr;37(4):5031-7.
- Zhang W, Khojasteh M, Cummins N, et al. 2016. Quantitative image analysis of PD-L1, CD8, CD3, CD68 and FoxP3 protein expression in lung and bladder cancer specimens by fully automated multiplex fluorescence immunohistochemistry. J Clin Oncol. May;34(15):11590.
- Hollander P, Kamper P, Smedby KE, et al. 2017. High proportions of PD-1+ and PD-L1+ leukocytes in classical Hodgkin lymphoma microenvironment are associated with inferior outcome. Blood Adv. Aug;1(18):1427-39.