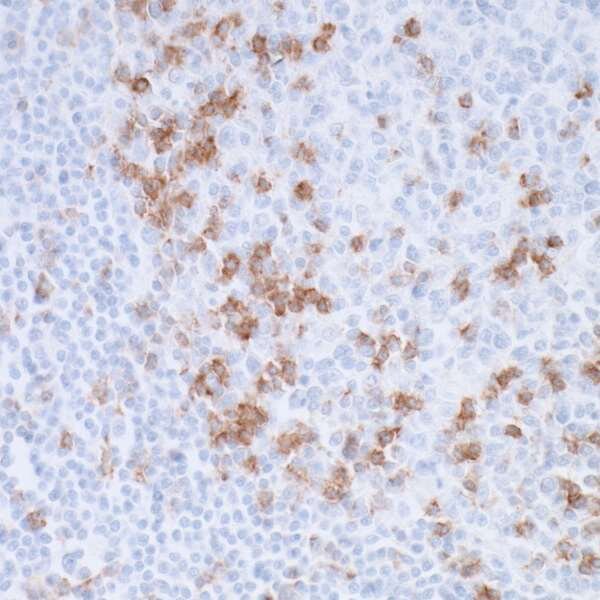
Programmed Cell Death-1 (PD-1)
Programmed cell death-1 (PD-1) is an immune checkpoint molecule that belongs to the CD28 family, which also includes CTLA-4. PD-1 is expressed on T cells and functions as a negative regulator of helper and effector T cell activity. Its ligands, PD-L1 and PD-L2, are members of the B7 family and are expressed by antigen presenting cells as well as tissue type-specific cells throughout the body and cancer cells. Binding of PD-1 to its ligands activates an intracellular signaling cascade that results in the dephosphorylation of the T cell receptor or of CD281, and the consequent reduction in T cell activity. In healthy individuals, this pathway tunes the immune system to protect against autoimmunity by suppressing effector T cells and promoting the activity of regulatory T cells (Tregs)2, 3.
PD-1 has been identified as an important regulator of anti-tumor immunity. In the cancer setting, the PD-1/PD-L1 pathway in particular has been implicated in suppressing the immune response that would otherwise lead to tumor clearance. Overexpression of PD-L1 by tumor cells is one of the mechanisms used by tumors to evade immune detection. T cells that attempt to enter the tumor microenvironment are rapidly inhibited when they encounter the PD-L1 expressed by the tumor. The PD-1 pathway was first understood to play a role in anti-tumor immunity in 2002, when a study in the murine P815 mastocytoma model showed that expression of PD-L1 by the tumor cells enhanced tumor growth and metastasis, while injection of a blocking antibody prevented this from occurring4.
Since then, the importance of the PD-1 pathway in anti-tumor immunity has been a major focus of research in the field and has led to remarkable advances in the way that cancer is treated clinically5. Several anti-PD-1 therapies have been approved by the FDA for the treatment of different solid tumors5. (Several anti-PD-L1 therapies are also FDA approved). Indeed, anti-PD-1 treatments were among the first wave of checkpoint blockade immunotherapies that have revolutionized the way that cancer is treated6. Cancers that can be treated with anti-PD-1 antibodies include melanoma, lung cancer, bladder cancer, ovarian cancer, lymphoma, squamous cell carcinoma, renal cell carcinoma, and more6–8. Some of these cancers, such as metastatic melanoma and ovarian cancer, were notoriously difficult to treat before the introduction of anti-PD-1 therapies.
Anti-PD-1 immunotherapy is sometimes given in conjunction with a CTLA-4-blocking antibody, a combination which can benefit patients independent of whether the tumor expresses PD-L19. Conversely, an early clinical trial in metastatic melanoma showed that for patients treated with anti-PD-1 antibody alone, the response rate is more than double for patients whose tumors express PD-L1 compared to patients whose tumors do not10.
However, not all patients whose tumors express PD-L1 will respond to anti-PD-1 therapy6, and some patients who initially respond will develop resistance to the treatment11. Other biomarkers that are being investigated to predict which patients will respond to anti-PD-1 immunotherapy include the immune signature of the tumor prior to treatment, including the ratio of effector CD8 T cells to Tregs, the expression of interferon gamma, or the amount of T cell infiltration into the tumor microenvironment12. An existing immune presence in the tumor is predictive of a better response to treatment6, and T cell exhaustion is one marker of resistance to treatment11. Despite the success of anti-PD-1 therapies in the clinic, these outstanding questions mean that more research is necessary to truly understand how anti-PD-1 and other checkpoint blockade immunotherapies can benefit the most patients.
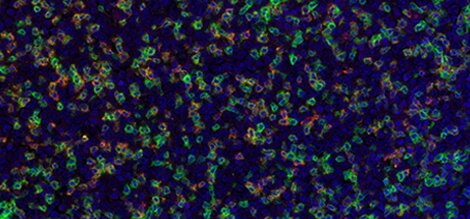
Detection of human CD3 (green), PD-1 (red), and PD-L1 (yellow) in FFPE tonsil by IHC-IF. Rabbit anti-CD3e recombinant monoclonal [BL-298-5D12] (A700-016), rabbit anti-PD-1 recombinant monoclonal [BLR076G] (A700-076), and rabbit anti-PD-L1 recombinant monoclonal [BLR020E] (A700-020). Secondary: HRP-conjugated goat anti-rabbit IgG (A120-501P). Substrate: Opal™. Counterstain: DAPI (blue).
References
- Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, Sasmal DK, Huang J, Kim JM, Mellman I, Vale RD (2017) T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 355:1428–1433 . doi: 10.1126/science.aaf1292
- Sharpe AH, Pauken KE (2018) The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol 18:153–167 . doi: 10.1038/nri.2017.108
- Bardhan K, Anagnostou T, Boussiotis VA (2016) The PD1:PD-L1/2 Pathway from Discovery to Clinical Implementation. Front Immunol 7:550 . doi: 10.3389/fimmu.2016.00550
- Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N (2002) Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci 99:12293–12297 . doi: 10.1073/pnas.192461099
- Iwai Y, Hamanishi J, Chamoto K, Honjo T (2017) Cancer immunotherapies targeting the PD-1 signaling pathway. J Biomed Sci 24:26 . doi: 10.1186/s12929-017-0329-9
- Callahan MK, Postow MA, Wolchok JD (2016) Targeting T Cell Co-receptors for Cancer Therapy. Immunity 44:1069–78 . doi: 10.1016/j.immuni.2016.04.023
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M (2012) Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. N Engl J Med 366:2443–2454 . doi: 10.1056/NEJMoa1200690
- Geng Z, Xiao Y, Zhu X-J, Ye C, Zhou J-F (2018) Anti-PD-1 therapy for clinical treatment of lymphoma: a single-arm meta-analysis. Oncotarget 9:35343–35355 . doi: 10.18632/oncotarget.26223
- Antonia SJ, Gettinger SN, Chow LQM, Juergens RA, Borghaei H, Shen Y, Harbison C, Chen AC, Ready N, Rizvi NA (2014) Nivolumab (anti-PD-1; BMS-936558, ONO-4538) and ipilimumab in first-line NSCLC: Interim phase I results. J Clin Oncol 32:8023–8023 . doi: 10.1200/jco.2014.32.15_suppl.8023
- Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen T-T, Berman DM, Wolchok JD (2015) Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol 33:1889–1894 . doi: 10.1200/JCO.2014.56.2736
- Wang Q, Wu X (2017) Primary and acquired resistance to PD-1/PD-L1 blockade in cancer treatment. Int Immunopharmacol 46:210–219 . doi: 10.1016/j.intimp.2017.03.015
- Melero I, Rouzaut A, Motz GT, Coukos G (2014) T-cell and NK-cell infiltration into solid tumors: a key limiting factor for efficacious cancer immunotherapy. Cancer Discov 4:522–6 . doi: 10.1158/2159-8290.CD-13-0985