Tyramide Signal Amplification (TSA)-Based Immunofluorescent Multiplex (mIF) Assays
Multiplex immunofluorescent (mIF) assays provide information on the expression levels of biomarkers. This protocol gives you the guidelines to optimize immunofluorescence assays.
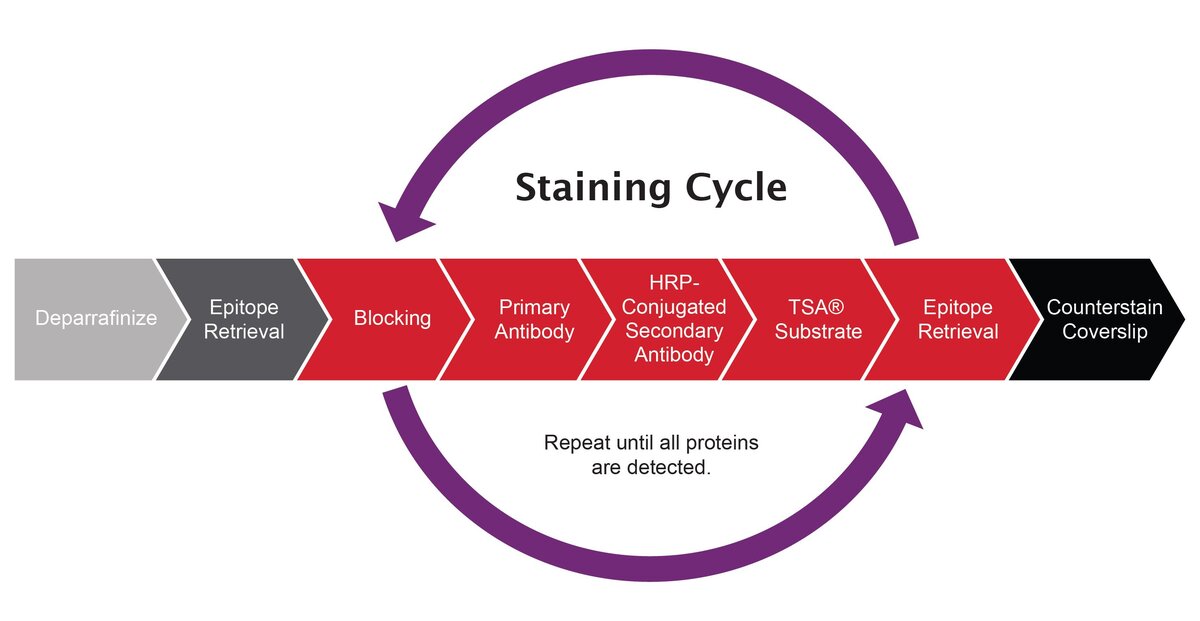
Figure 1. mIF Staining Cycle. In the tissue staining process, the primary and secondary antibodies corresponding to the first target of interest are deposited. The antibodies are removed from the tissue using heated washes (also referred to as epitope retrieval) and the staining process is repeated for a subsequent target of interest. The process is repeated until all targets have been labeled. The last steps before imaging are applying DAPI to counterstain and applying a cover slip. Repeat until all proteins are detected.
Introduction to Multiplex Immunohistochemistry
Multiplex immunohistochemistry allows for the detection of multiple antigens on the same tissue section. It provides colocalization and spatial orientation of proteins, which facilitates an accurate determination of the target's subcellular localization, identification of multiple cell types, and resolution of the relative proximity of biomarkers. More specifically, multiplex immunofluorescent (mIF) assays provide information on the expression levels of biomarkers while increasing the number of biomarkers that can be visualized simultaneously on a single slide. Thus, the advantage of mIF assays can be expressed as better information about the tissue microenvironment while conserving the sample.
The use of TSA® systems in mIF provide advantages over traditional methods. The advantages include the ability to use antibodies from the same species and to detect low-abundance proteins. Tyramide signal amplification uses horseradish peroxidase (HRP)-conjugated secondary antibodies to covalently link a fluorophore to the tissue. Amplification is the result of significant quantities of fluorophore labeled tyramide that are deposited at the site of the protein of interest. This high fluorophore density results in an intense signal.
Guidelines for the Optimization of Immunofluorescence Multiplex Assays
Success with any mIF assay requires the use of the highest quality immunofluorescent-validated antibody for each protein of interest. Each antibody must be selected and tested with great care. As a rule of thumb, if a singleplex staining using DAB substrate does not produce specific staining with a low-to-zero background, then complications will arise during multiplex procedures because the tyramide amplification process will magnify deficiencies.
Many factors contribute to a poor signal-to-noise ratio. These factors include tissue autofluorescence, low amounts of the target protein, inefficient blocking, inefficient rinsing, and suboptimal concentrations of primary and secondary antibodies. If not well controlled, each of these factors can compromise the quality of the assay.
To overcome these barriers, observe these practical guidelines to optimize each of four key parameters:
- The concentration of the primary antibody
- The best fluorophore/antibody pairing
- The concentration of Opal
- The best order of target staining
For demonstration purposes, the graphs presented below were produced from our experience using CD3e, CD8, CD20, CD68 and PD-L1 as primary antibodies and Opal as the detection system.
1. Optimal Concentration of the Primary Antibody
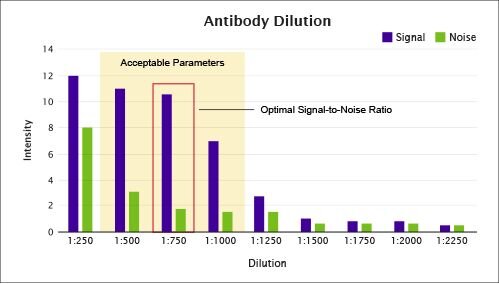
Figure 2. A high-signal-to-noise ratio is key to achieve success with mIF assays.
Optimizing the primary antibody concentration requires (1) knowledge of the staining pattern of antibodies as detected by DAB and (2) optimization of the signal using TSA® substrate. The user must determine the appropriate dilution of the primary antibody in order to produce a strong signal and a favorable signal-to-noise ratio.
In Figure 2, dilutions between 1:500 and 1:1000 provide acceptable parameters with 1:750 providing the optimal signal-to-noise ratio (10:1). Although 1:250 produces a signal of higher intensity, the corresponding signal-to-noise ratio (1.5:1) is unacceptable.
In contrast, dilutions below 1:1250 do not provide a sufficiently strong signal. It is important to perform this optimization process for each primary antibody that is to be used in the multiplex. The optimal dilution is the one that (1) replicates the DAB staining pattern, and (2) exhibits the highest signal-to-noise ratio with TSA® fluorophore substrates.
2. Best Flurophore/Antibody Pairing
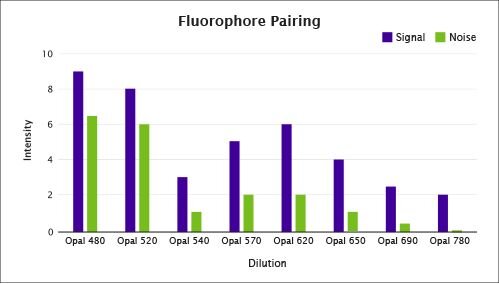
Figure 3. Correct pairing between the fluorophore and the primary antibody can assist in balancing signals between proteins of different abundance.
Optimal results are obtained when the signal corresponding to each protein is distinguishable from the others, with no significant overlap between channels – each channel being composed of a filter set leading to one color associated with a given primary antibody. This is especially important for proteins that are being detected in adjacent channels where spectral overlap from a very high signal in one channel may compromise the detection of another protein from the adjacent channel. Thus, determining the right pairing between the primary antibody and the fluorophore at satisfactory signal-to-noise ratios is critical.
In Figure 3, the Opal 620 fluorophore exhibits a strong signal with an optimal signal-to-noise ratio. Although Opal 480 and 520 fluorophores exhibit a strong signal, they exhibit higher inherent noise due to tissue autofluorescence. In most instances, the detection of low abundance proteins will require balancing the fluorophore's ability to be detected by the imaging system with the tissue autofluorescence observed with the filter set used to image that fluorophore.
3. Optimal Opal Concentration
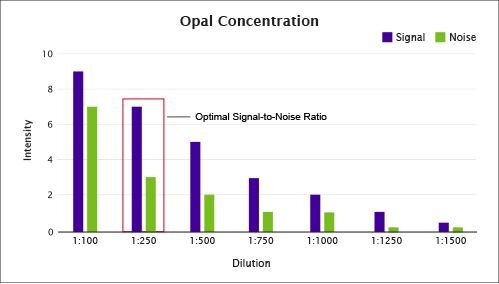
Figure 4. Determining the optimal Opal concentration can reduce background and assist in balancing signals.
Too much tyramide (TSA®) substrate can lead to excess fluorophore deposition on the tissue creating excessive background. In Figure 4, the 1:250 dilution exhibits the best signal-to-noise ratio. It is important to note that 1:500 dilution might be used in cases where it was necessary to reduce the signal in order to balance the detection of proteins to be imaged in adjacent channels.
4. Best Order of Target Staining
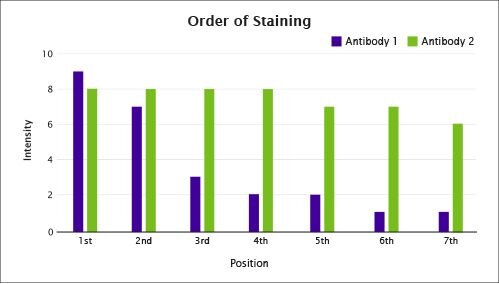
Figure 5. The correct order of staining can be the difference between a robust signal and a non-existent signal.
The process of mIF using TSA® as the detection system requires sequential staining in which antibody binding, fluorophore deposition and antibody removal are repeated until all targets are detected. The successive exposure of the tissue to heat induced epitope retrieval (HIER) may lead to either the degradation or enhancement of epitopes. The impact of HIER cycles on any given epitope cannot be predicted. Therefore, it is important to determine the order of staining to get the best results.
To optimize the order of staining, a singleplex stain is performed with as many HIER cycles as there are epitopes to detect. Importantly, this is done at the optimal antibody concentration and fluorophore/antibody pairing, as determined using the guidelines mentioned above.
Figure 5 provides an example of how to choose the preferred staining order. In order to determine which antibody will be stained in the 3rd position for a multiplex of seven protein targets, two HIER steps would be performed, followed by a singleplex stain, and then the four remaining HIER steps. Antibody 1 exhibits decreased signal after the second antigen retrieval. Thus, Antibody 1 must be used during the first two cycles, as the signal would be too low in subsequent stainings. In contrast, the signal produced from Antibody 2 is only marginally influenced by repeated HIER, and it can be used for any of the 7 staining cycles.
Step-by-Step Protocol for mIF Assays: Formalin-Fixed, Paraffin-embedded tissues and cell blocks
Once the four key factors mentioned above are optimized, the following protocol can be used to perform mIF assays with 3 or more targets. The use of 3-5μm paraffin sections on charged slides is recommended.
Materials
- Xylene
- 100% Reagent alcohol-histology grade
- Endogenous Peroxidase Block Solution: 0.9% Hydrogen Peroxide in Methanol
- Epitope Retrieval Solutions:
- Citrate Buffer pH 6.0. Measure 2.94 g Tris-sodium Citrate (dehydrate) volume to 1 Liter with distilled water and pH to 6.0.
- Tris Buffer pH 9.0. Measure 1.21 g Tris Base and 0.37 g EDTA volume to 1 Liter with distilled water and pH to 9.0.
- 1x Tris-Buffered Saline (TBS), 0.1% Tween® 20 (TBST)
- Blocking Solution: 20% Normal Goat Serum in PBS
- IHC Antibody Diluent: 50mM TBS, 1% BSA
- HRP-conjugated Secondary Antibodies: Anti-mouse A90-116P, Anti-rabbit A120-501P
- Fluorophore-conjugated Tyramide Signal Amplification (TSA®)
- DAPI nuclear counterstain
- Slide mounting media
Slide Preparation
- Xylene - 3 changes for 5 minutes each. Agitate 10 times up and down.
- 100% Reagent Alcohol - 3 changes for 5 minutes each. Agitate 10 times up and down.
- 0.9% Hydrogen Peroxide in Methanol 40 minutes. Agitate 10 times up and down.
- Distilled water 2 X 5 minutes. Agitate 10 times up and down.
- Epitope retrieval (Refer to Bethyl Antibody datasheet for recommended retrieval buffer). Heat epitope retrieval buffer to 92-96 °C. Place slides in epitope retrieval buffer for 20 minutes. Cool on benchtop for 20 minutes. Drain epitope retrieval buffer and add distilled water to the container.
- Circle tissue with hydrophobic barrier pen. Take care to prevent the samples from drying out.
Staining Cycles
Cycle #1 (1st target)
- Blocking: Incubate tissue in 20% Normal Goat Serum or serum (NS) matched to the host of the secondary antibody for 20 minutes at RT.
- Primary Antibody: Remove Blocking Buffer and add primary antibody diluted in IHC antibody diluent (Refer to Bethyl data sheet for recommended dilution range. Optimal working dilutions should be determined experimentally by the investigator). Incubate for 20 minutes at RT.
- Rinse with TBST - 3 changes for 5 minutes each. Agitate 10 times up and down.
- HRP-conjugated Secondary: Add 1:400 diluted HRP-conjugated secondary (goat anti-rabbit: A120-501P or goat anti-mouse: A90-116P) and incubate for 20 minutes at RT.
- Rinse with TBST - 3 changes for 5 minutes each. Agitate 10 times up and down.
- TSA® Substrate: Dilute fluorophore-conjugated TSA® and incubate according to manufacturer's instructions.
- Rinse with TBST- 3 changes for 5 minutes each. Agitate 10 times up and down.
- Epitope retrieval: For the next cycle, repeat epitope retrieval with the buffer recommended on the datasheet of the primary antibody (refer to step #5).
Cycle #2 with 2nd target
- Blocking: Incubate tissue in 20% Normal Goat Serum or serum (NS) matched to the host of the secondary antibody for 20 minutes at RT.
- Primary Antibody: Remove Blocking Buffer and add primary antibody diluted in IHC antibody diluent (Refer to Bethyl data sheet for recommended dilution range. Optimal working dilutions should be determined experimentally by the investigator). Incubate for 20 minutes at RT.
- Rinse with TBST - 3 changes for 5 minutes each. Agitate 10 times up and down.
- HRP-conjugated Secondary: Add 1:400 diluted HRP-conjugated secondary (goat anti-rabbit: A120-501P or goat anti-mouse: A90-116P) and incubate for 20 minutes at RT.
- Rinse with TBST - 3 changes for 5 minutes each. Agitate 10 times up and down.
- TSA® Substrate: Dilute fluorophore-conjugated TSA® and incubate according to manufacturer's instructions.
- Rinse with TBST- 3 changes for 5 minutes each. Agitate 10 times up and down.
- Epitope retrieval: For the next cycle, repeat epitope retrieval with the buffer recommended on the datasheet of the primary antibody (refer to step #5).
Cycles #3-8
- Repeat steps 15-22 as needed for the desired number of targets in multiplex, up to 8
Counterstain and Mount
- Rinse slides in dH2O
- Rinse slides in TBST
- Incubate slides in DAPI solution according to manufacturer’s protocol
- Rinse slides with TBST for 5 minutes
- Rinse slides in dH2O for 5 minutes
- Mount with preferred mounting media
Imaging Recommendations
Multiplex IF can be imaged on multiple systems. We recommend using fluorescent slide scanners. While standard epi-fluorescent and confocal microscopes can be adapted to image multiplex experiments, the ability to image entire tissue sections and automated controls make slide scanners the preferred instruments. If fluorophores exhibit overlapping spectra that do not allow for complete isolation by filters, some form of multispectral imaging will be needed.
The type of imaging instrument is dependent on the number of targets and whether your set of fluorophores exhibit spectral overlap. The list below includes some instrumentation for multiplex (not an exhaustive list).
3-color or less (2 antibodies + DAPI):
- Standard Research grade fluorescent microscopes
- Confocal microscopes
- Mantra
- Aperio Versa slide scanners
- Zeiss Axioscan
- 3DHisTech Panoramic slide scanners
- Vectra, Vectra 3, and Vectra Polaris
4-color or more (3 or more antibodies + DAPI):
- Confocal microscopes
- Mantra
- Aperio Versa slide scanners
- Zeiss Axioscan
- 3DHisTech Panoramic 250 slide scanners
- Vectra, Vectra 3, and Vectra Polaris
Fluorophores whose spectra overlap:
- Advanced confocal systems
- Mantra
- Vectra, Vectra 3, and Vectra Polaris
Troubleshooting
Low signal
- Ensure antibody is suitable for IHC by DAB detection
- Titer primary antibody using TSA substrate
- Increase secondary concentration
- Increase TSA substrate concentration
- Increase TSA incubation times
- Test different HIER methods
- Test primary on a known positive tissue
- Reoptimize order of antibodies
High signal
- Titer primary antibody using TSA substrate
- Decrease secondary concentration
- Decrease TSA substrate concentration
- Decrease TSA incubation times
High background
- Perform Peroxidase block prior to staining
- Titer antibody components
- Increase rinse times and number of rinse exchange
- Filter all buffers to ensure no particulates contact the tissue
- Switch blocking buffers
Excess TSA signal (fluorescent speckles or particulates on tissue)
- Increase rinse times
- Rinse slides with more vigorous motion
- Dilute TSA substrate
- Dilute HRP-conjugated secondary
Signal for one antigen overlaps with another antigen
- Ensure HIER was used to strip antibodies before beginning next cycle of staining
- Image using the proper filters for each fluorophore
- If fluorophore spectra overlap, use multispectral imaging
Tissue autofluorescence
- Use multispectral imaging
- Shift fluorophores from the green to red portion of the spectrum
FAQs
What do I need to get started with mIF?
mIF requires high quality IHC validated primary antibodies, HRP-conjugated secondary antibodies, tyramide detection fluorophores, and an imaging system capable of separating the fluorophores being used in the assay.
Can I use any primary?
Primary antibodies of any host species can be used in mIF, even if the antibodies are from the same host species. It is advisable to begin developing mIF with highly validated IHC antibodies. Bethyl offers an extensive portfolio of IHC validated antibodies. Antibodies from other companies are compatible with Bethyl antibodies but will need to be validated by the researcher.
What types of samples do I need?
The most common mIF sample is formalin fixed paraffin embedded tissue (FFPE). Since mIF with tyramide detection uses multiple rounds of heat induced epitope retrieval, non-FFPE tissue tends to degrade during the assay.
Where do I purchase tyramide detection fluorophores?
Bethyl antibodies are validated for mIF with the Opal detection system from Akoya Biosciences. Use of other tyramide reagents will require independent validation by the researcher.
Do I have to perform any optimization steps?
The mIF protocol outlines the basic optimization parameters. The recommendations given for mIF applications are starting points for assay development. Further optimization of antibodies will be needed using your own tissue. Differences in tissue fixation, epitope retrieval buffer, instruments used for epitope retrieval, and technical skill can impact the mIF assay. The mIF protocol outlines the basic optimization parameters.
Why do I observe signal in singleplex stains but not during multiplex stains?
Order of staining can impact the detection of antigens. Multiple rounds of epitope retrieval can destroy some epitopes. In this case, antibodies recognizing these markers need to be used early in the staining order. Bethyl validated mIF panels will have established an initial staining order. This order should be confirmed on your own tissue before proceeding mIF studies.
Do you have any recommendations for antigens that are localized to the same cell type within the tissue?
Fluorophore pairings should be carefully considered when staining for markers that occur in the same cell type, especially if those markers are localized to same subcellular location. In these cases, it is advised to use fluorophores whose spectra are non-overlapping.
What do I need to perform heat induced epitope retrieval (HIER)?
Bethyl uses steamers for HIER because this method is widely used. Other methods, such as microwave treatment, may be used but will require independent validation by the researcher.
How many targets can I detect in one tissue sample?
The number of targets detectable is dependent on available tyramide fluorphores and the imaging system. Several slide scanners such as the Vectra Polaris offer the capablilty to perform a 9-color scan (8 immunostained markers + DAPI).