Flow Cytometry
Introduction to Flow Cytometry
Flow cytometry is a technique commonly used by researchers and clinicians to analyze properties of individual cells based on the presence of specific extracellular or intracellular targets. These targets may be proteins, post-translational modifications, nucleic acids, exosomes, and more.
This technique interrogates suspensions of single cells as they pass through a laser at high speed. The fluorescence and light scattering from individual cells can then be used for subsequent analysis, determining the unique properties of the cells at both the single-cell and population levels.
Flow cytometry has many practical applications, including the detection and measurement of protein expression, cell cycle, and health status, and the identification and characterization of cells within a heterogeneous sample for preclinical and translational research purposes.
What is Flow Cytometry Used For?
Flow cytometry is a biological laboratory technique that is used to detect, identify, and count specific cells within a sample. The method also can identify particular components that make up the target cells, based on physical characteristics and antigens that are present on the surface or within a cell. Flow cytometry can be used to detect fluorescent antibody-tagged proteins, nucleic acids, and other subcellular components, fluorescent and fluorescently-tagged proteins, nuclear stains, dyes that measure cell membrane permeability and cell viability, and more.
Based on this, flow cytometry can be used for many different applications, including cancer detection and treatment, genetics, molecular and cellular biology, cytokine analysis, and more.
How Does Flow Cytometry Work?
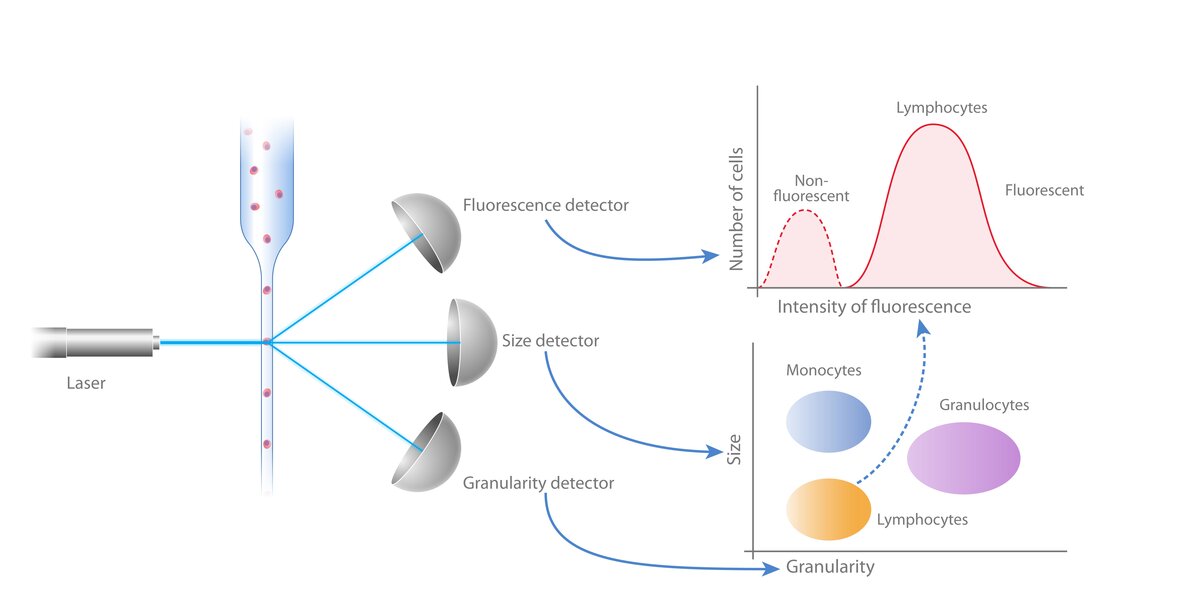
To perform flow cytometry, a sample containing suspended cells or particles is loaded into a flow cytometer. Samples are transported to a flow cell, before being injected at high speed to a laser. These individual cells then pass single file through a laser beam, causing fluorescence of samples and light scattering.
The light scattering information of each individual cell - the forward angle scatter (FSC), a measure of cell size, and side angle scatter (SSC), a measure of cell granularity - and fluorescence emission are collected and processed by a detector, providing quantitative information about each cell in the sample. Flow cytometry experiments can be designed to detect any number of cellular markers, from as few as 1-4 fluorescent markers in the most simple experiments to up to 50 or more depending on the number of lasers and filters in the flow cytometer and the availability of compatible antibodies.
Extensions of Flow Cytometry Methodology
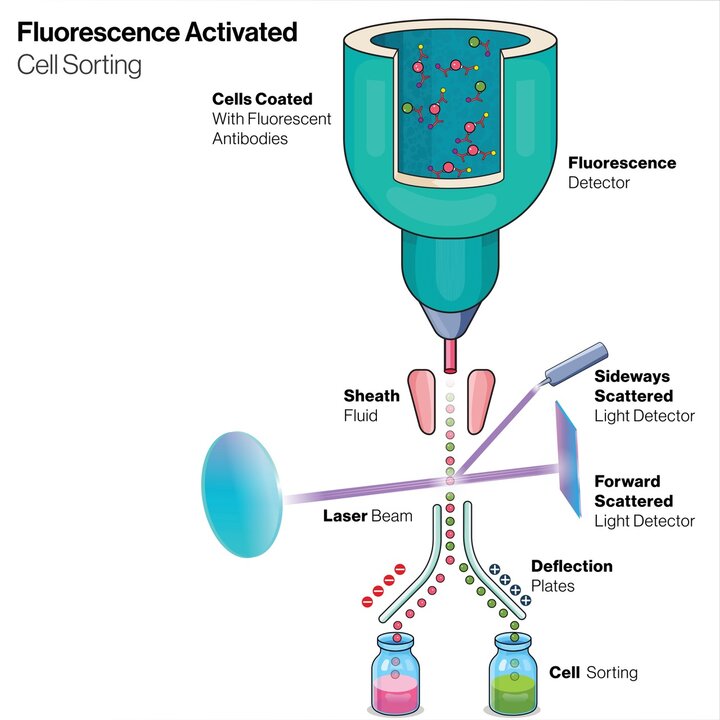
A related technique called Fluorescence Activated Cell Sorting (FACS) can be used to subsequently sort the cells based on specific properties for use in downstream assays. Leveraging the principles of flow cytometry, FACS enables the identification and sorting of cells based on their unique fluorescence profiles, providing researchers with the ability to selectively collect cells of interest with purity and efficiency. By coupling fluorescent labeling techniques with high-speed sorting capabilities, researchers gain even more information on specific cell populations that were identified within the parent sample.
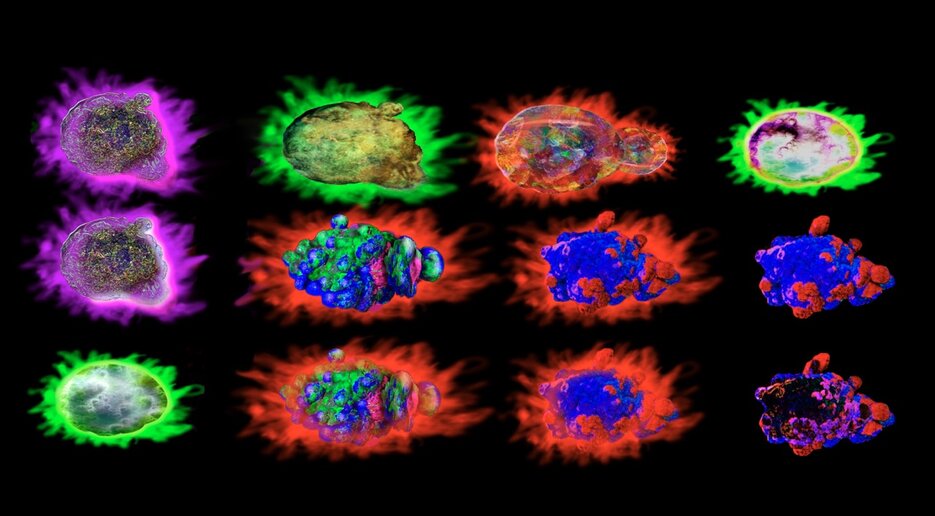
Imaging flow cytometry is another modern adaptation of this technique. In addition to quantifying cell populations, this sophisticated technique adds detailed imagery provided by microscopy. By capturing multiple high-resolution images of each cell as it flows through the instrument, imaging flow cytometry empowers researchers to visualize cellular morphology, subcellular structures, and spatial relationships between labeled subcellular components, all while maintaining the throughput necessary for analyzing large sample sizes.
Protocols for Flow Cytometry
- Indirect Intracellular Staining of Cultured Cells Grown in Suspension
- Fixation & Permeabilization of Whole Blood with Red Blood Cell Lysis
- Staining of Intracellular & Nuclear Antigens by Flow Cytometry
- Cell Surface Staining for Live Cells Using Flow Cytometry
- Application note: Flow Cytometry Protocols for Intracellular & Extracellular Targets
Flow Cytometry Resources
Flow Cytometry FAQs
What does flow cytometry detect?
How many cells are needed for flow cytometry?
When should you use flow cytometry?
Who uses flow cytometry?