Focus on the Cell Cycle
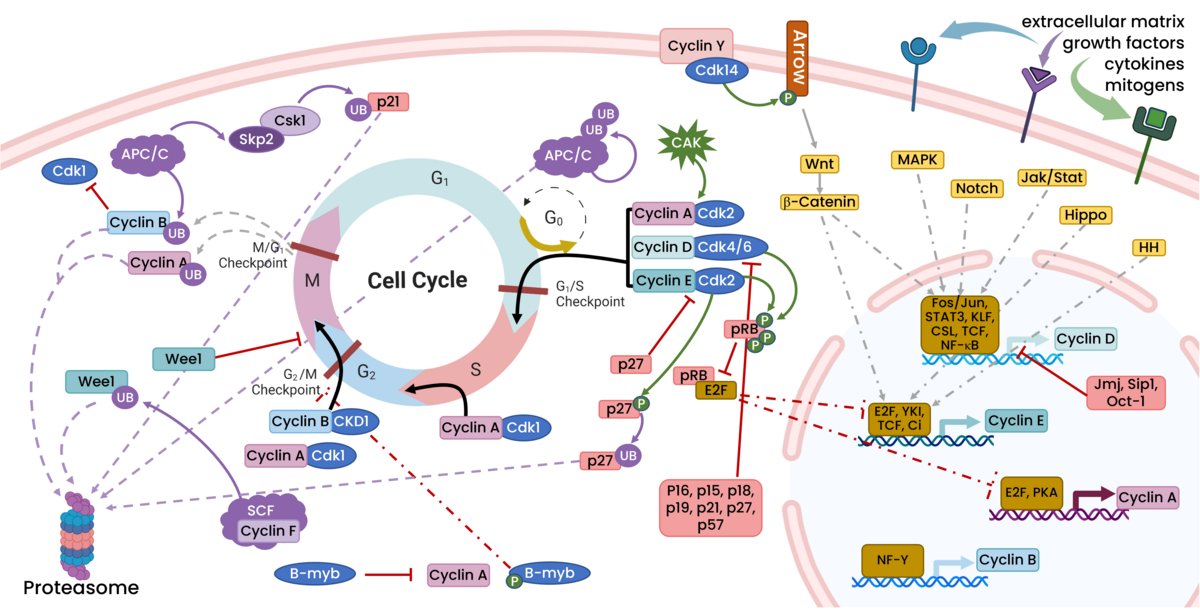
Cell proliferation is a highly regulated process. There are multiple checkpoints, regulators, and inhibitors that play an important role in determining whether proliferation proceeds normally or is impeded. The cell cycle is divided into four phases: G1, accumulation and generation of the materials needed for a cell to proliferate; S, also known as synthesis, where DNA replication occurs; G2, more material accumulation, organelle replication, and preparations for mitosis; and M, mitosis, where duplicated DNA and organelles are precisely divided resulting in two identical daughter cells. Additionally, not all cells are undergoing cell division or preparing for proliferation; these quiescent cells are located in G0 temporarily or permanently.
As the cell transitions between each of the phases of the cell cycle there are checkpoints to ensure that everything in the cell is approved to move on to the next phase. If there are any defects, like DNA damage, DNA actively undergoing replication, inappropriate cell size, etc, the cell can stall at the transition until the errors are corrected. There are many forms of regulation layered across these checkpoints most of which revolve around the presence of cyclins and cyclin dependent kinases. Various growth factors, stimuli, and inhibitory factors regulate the expression of cyclins and cyclin dependent kinases, many of which are illustrated in the figure below.
When DNA Repair Goes Wrong
Within the genome there exist short, repeating segments of DNA - motifs of, typically, one through six base pairs that repeat five to 50 times. These segments of DNA are known as microsatellites and comprise approximately 3% of the human genome, in both coding and noncoding regions of the genome. Microsatellites are particularly susceptible to accumulating mutations during DNA replication.
The Speed Limit of the Cell Cycle
Proper regulation of the cell cycle is a critical process for normal cell biology, and the loss of control (for example, through mutations that produce abnormal CDK-cyclin complexes) leads to uninhibited cell growth and cancer.
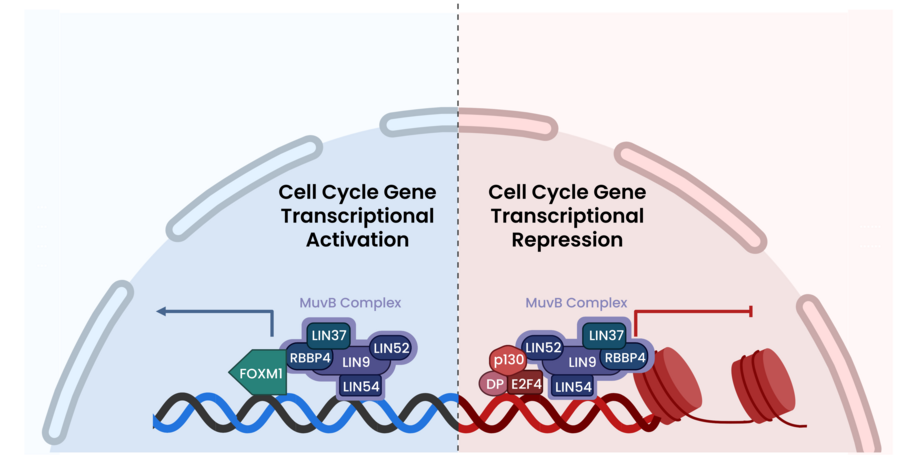
MuvB nucleosome binding is a critical regulator of cell cycle gene transcription repression
The MuvB complex is a critical regulator of cell cycle gene transcription. Alterations in binding partners at different stages of the cell cycle activate or repress gene transcription. This study found that MuvB transcriptional repression is dependent on nucleosome binding in the 1+ position.
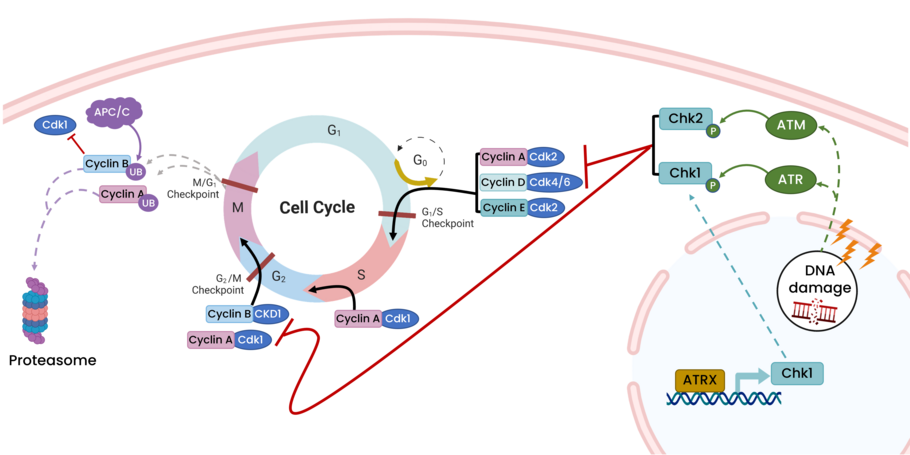
ATRX aids in cell cycle regulation through expression of Chk
ATRX is a chromatin remodeler that plays a critical role in transcriptional regulation of cell-cycle control genes. In this study, the authors identified Chk1 as a key regulator in the ATRX pathway in glioma. Loss of ATRX is a common occurrence in glioma and increases radiation sensitivity. Upstream inhibitors of Chk1, resulted in increased survival in a mouse brain cancer model and decreased cell proliferation in glioma cell lines.
Ran Proteins in Health and Disease
Ras-related nuclear protein (Ran), a member of the Ras superfamily of small GTPases, plays a critical role in the transport of macromolecules between the nucleus and the cytoplasm that occurs through nuclear pores. More recently, research has shown that Ran participates in the coordination of mitosis, with specific roles in mitotic spindle assembly, centrosome duplication, microtubule dynamics, and chromosome alignment.
Microtubule-Associated Proteins (MAPs): Life’s Mini Conveyor Belts
As their name suggests, microtubule-associated proteins (MAPs) are a family of proteins that reversibly interact with microtubules, a critical component of the cellular cytoskeleton.
Role of Kinesin Molecular Motor Proteins in Cancer
Kinesin superfamily proteins, or KIFs, are microtubule-dependent molecular motors whose movement is critical to a variety of cellular processes including mitosis, meiosis, and axonal transport.
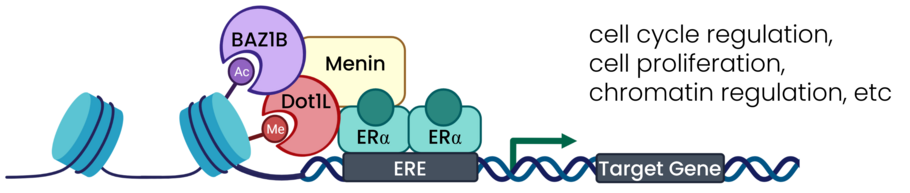
Novel combinatorial breast cancer treatment strategy targets Dot1L, menin, and BAZ1
Estrogen receptor alpha (ERα) is a nuclear transcription factor responsible for the regulation of many cell cycle, proliferation, and differentiation genes, especially in breast tissue. Dysregulation of ERα contributes to the formation of breast cancer and is a marker used to determine whether the cancer would be responsive to endocrine therapy. However, many ERα+ breast cancers are unresponsive to endocrine therapy or develop resistance to this treatment strategy. The authors in this paper identify novel combinatorial treatment strategies targeting Dot1L, menin, and BAZ1B.
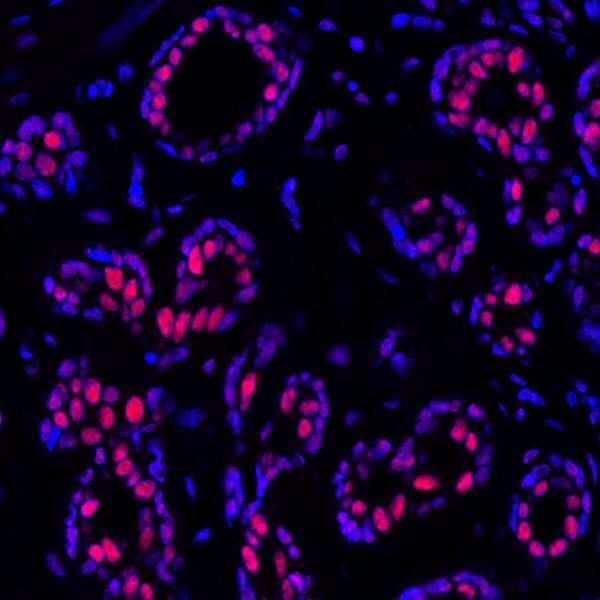
PBRM1 Joins p53 To Impact Kidney Cancer
p53 is a well-known tumor suppressor protein which suppresses cancer by regulating the cell cycle. It is encoded by the gene, TP53. A key component of p53’s suppressor activity is modulated through post-translational modifications, namely acetylation.
Self-Renewal
The concept of self-renewal applies to adult pluripotent stem cells as well as embryonic stem cells. Self-renewal allows a cell to maintain itself in an undifferentiated state until it receives signals to commit to a particular lineage.
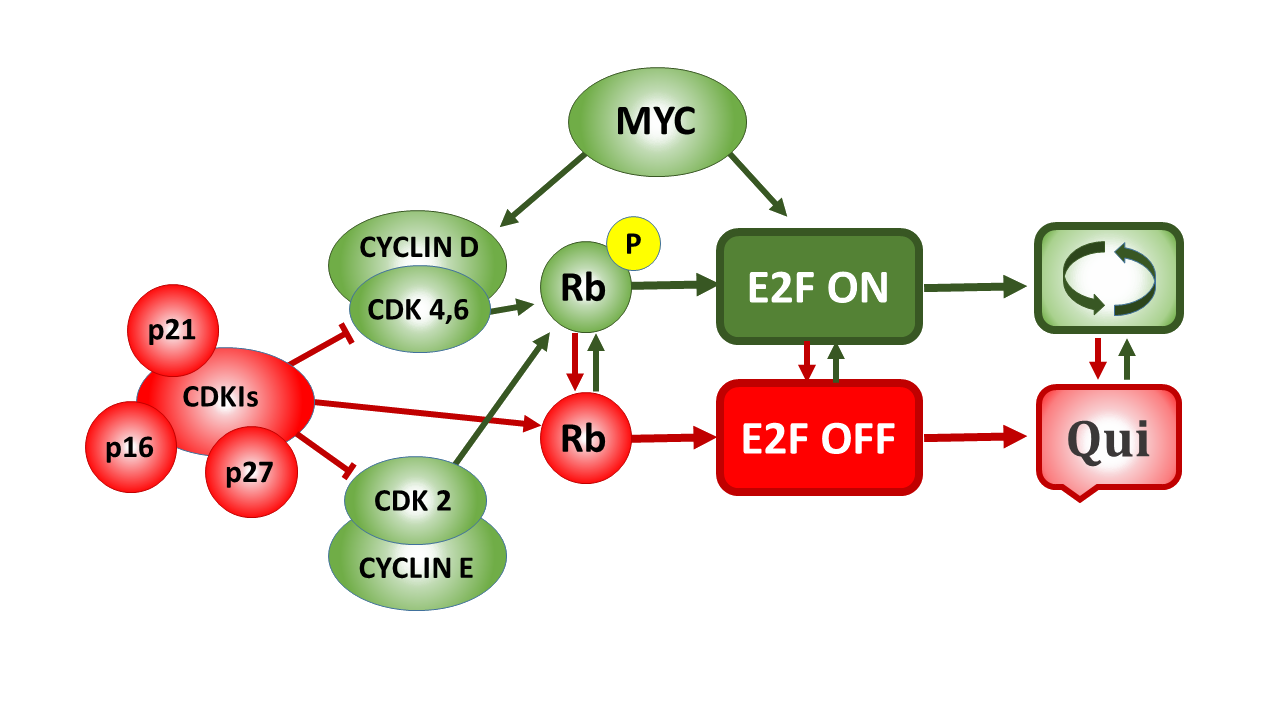
The Rb—E2F Switch: Regulation of Cellular Quiescence
Cell cycle progression and proliferation have been well-studied, while much less attention has been given to cellular quiescence corresponding to the G0 phase of the cell cycle. Recent studies have determined that G0 is not a single state, but a spectrum of quiescent states associated with an active transcriptional program. In a new study, Guang Yao and colleagues examined the factors that control entry into and exit from quiescence and commitment to cell cycle progression.