Beyond mAbs: The Unrealized Potential of VHH Antibodies
When Georges Köhler and César Milstein cracked the code of fusing B cells with myeloid cells to create hybridomas in 1975, they didn’t just make a scientific breakthrough—they propelled the possibilities of personalized medicine into the future. This method of producing monoclonal antibodies (mAbs) to target specific antigens was so revolutionary it earned them the Nobel Prize in 1984.
Since then, the FDA has approved over 100 mAb products for a wide range of applications, including cancer, autoimmune disorders, and infectious diseases. By 2021, nearly one in five new FDA approvals used mAb technology and by early 2024, an additional 130 antibody therapeutics were in late-stage clinical trials worldwide.1,2
In this article, we will explore the differences between monoclonal antibodies, VHH antibodies (variable heavy chain of heavy-chain-only antibodies), and other non-canonical antibody types, compare antibody engineering methods, and discuss the possibilities of mAbs and VHH in the context of antibody engineering goals and applications.
A Small Discovery with a Big Impact
Fifteen years after mAbs were unlocked, Raymond Hamers-Casterman and his colleagues made another groundbreaking discovery. They found that animals from the Camelidae family, such as camels, llamas, and alpacas, produce a unique class of small antibodies in addition to conventional immunoglobulins.
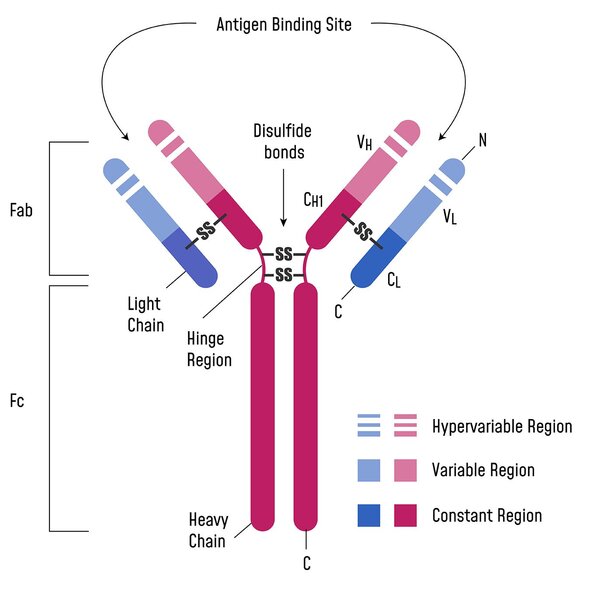
These camelid heavy chain-only antibodies are lacking the CH1 domains typically needed for light chain pairing. The full-length (VHH-hinge-Fc) protein found in camelid serum is termed a heavy-chain only antibody, or hcAb. The VH domain from these hcAb are VHH.3
Once researchers isolated the VHH domains, they found that these single-domain antibodies had better solubility and stability than conventional full-length monoclonal antibodies.4,5 It was also discovered that VHH naturally possess longer complementarity determining region 3 (CDR3) loops that enable improved access to hard-to-reach epitopes.6,7 VHH are able to mature toward longer CDR3 lengths because they are unconstrained by the paired VL present in a mAb.
VHH have since been harnessed extensively for a variety of research and clinical applications, largely due to their small size, high affinity and stability, low immunogenicity, enhanced solubility, and good tissue penetration. In 2019, the FDA approved its first VHH therapeutic, with four more approvals following suit. Today, VHH antibody-based therapeutics are being evaluated in 22 clinical trials worldwide.8
Successful diagnostic and therapeutic antibody engineering depends on discovering antibodies with high affinity and specificity for the target and low off-target toxicity. Advances in antibody engineering approaches allow the finetuning of qualities like affinity, size, and valency to optimize biodistribution and pharmacokinetics for specific applications. And, as technology continues to advance, new frontiers in personalized medicine will emerge, offering endless possibilities for those willing to explore.
Drilling Down: The Antibody Landscape
Through antibody engineering and recombinant methods, it is possible to harness the modular nature of antibodies to better suit your application. In addition to improving the ease of access to challenging epitopes, the use of smaller antibodies can facilitate higher yields, faster production times, and lower costs.9 There are several types of non-canonical antibodies, ranging from molecular weights of 14–105 kDa:
Table 1: Size and Structural Comparison of Antibodies
| Monoclonal antibody (mAb) | Camel IgG2/IgG3 heavy-chain-only antibody (hcAb) | Fragment antibody (Fabs) | Single-chain fragment variable antibody (ScFv) | Variable domain of heavy chain from hcAb antibody | |
|---|---|---|---|---|---|
| Structure |
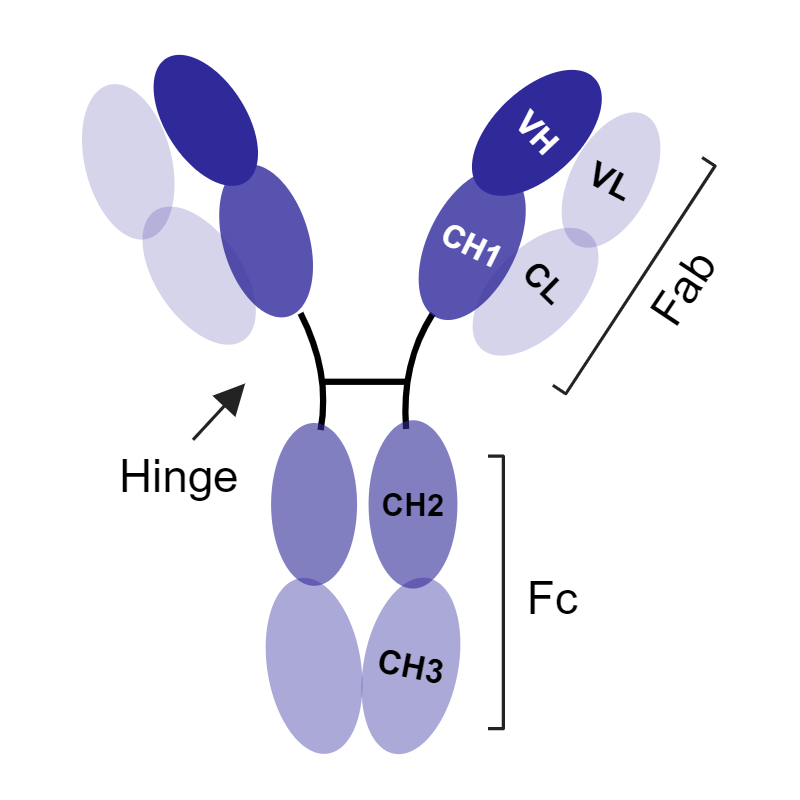
Full length antibodies with two heavy chains and two light chains |
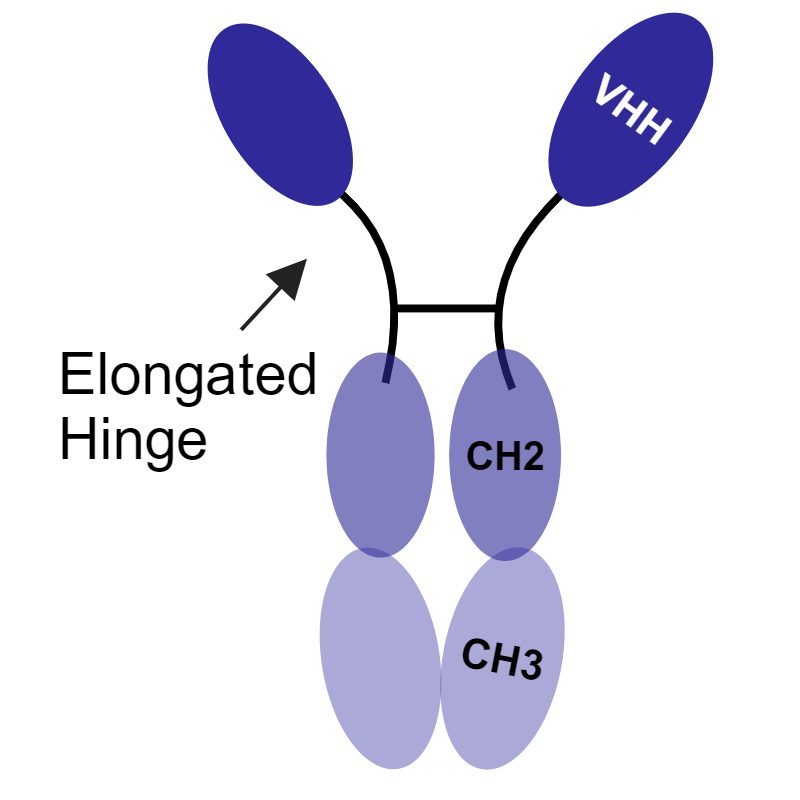
Absence of CH1 domains; missing light chains |
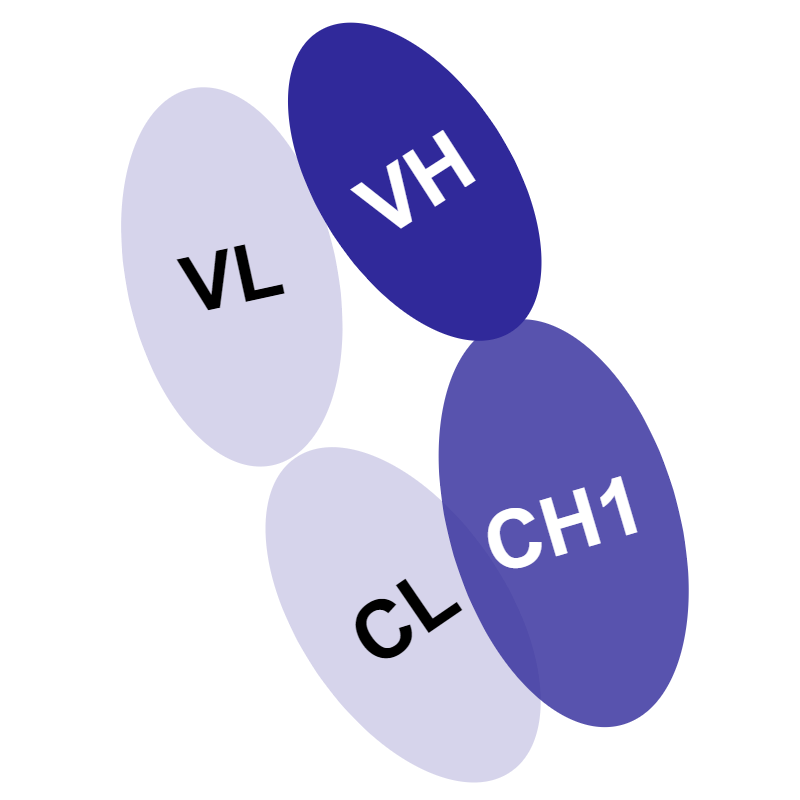
Variable part and constant heavy domain 1 from both light and heavy chains (VH + CH1 and VL + CL) |
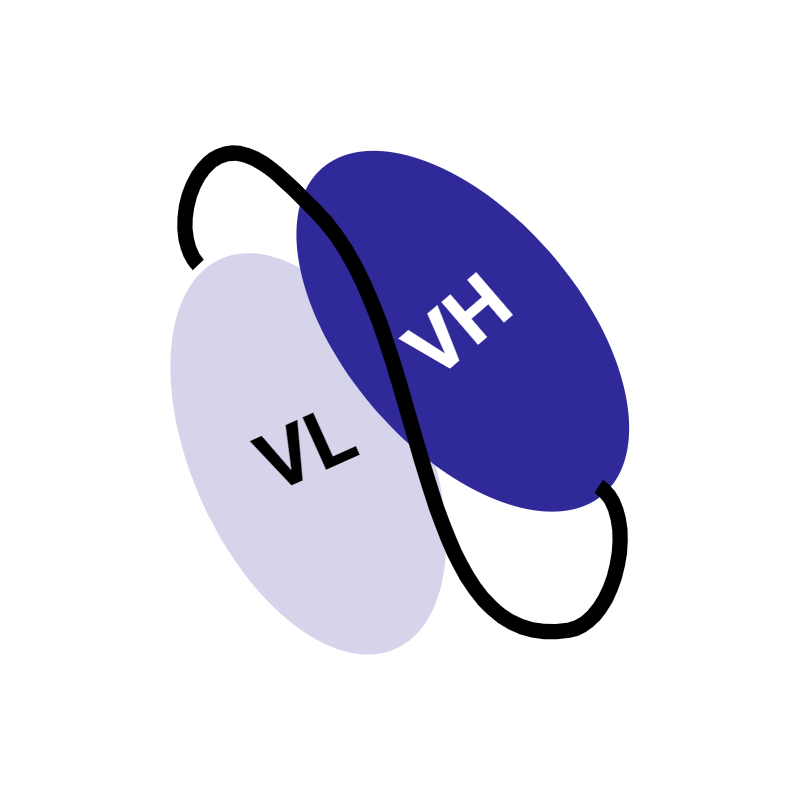
Variable heavy and variable light domain (VH + VL) plus linker |

The variable domain of HCAbs |
| Molecular mass | 150 kDa | 105 kDa | 55 kDa | 28 kDa | 14 kDa |
Now, let’s take a closer look at the key biophysical differences between mAbs and VHH, the smallest naturally occurring intact antigen binding domain.10
| Monoclonal antibodies (mAbs) | Variable heavy domain of heavy chain (VHH) | |
|---|---|---|
| Engineering methods | Hybridomas, recombinant DNA technology, single B cell screening, phage display | Animal-free methods: recombinant DNA technology, phage display |
| Size | Larger, allowing for strong binding of larger antigens | Smaller, can bind to concave and hidden epitopes like enzyme active sites and cryptic viral epitopes that larger antibodies are unable to access11 |
| Solubility | More variable. | Increased hydrophilicity compared to full-length mAbs.12 |
| Stability | Lower | Higher; outperforms mAbs in thermal, chemical, and pH stability13 |
| CDR3 loop length | Length depends on species, typically 6-20 amino acids | Length depends on epitope, typically 8-25 amino acids |
| Serum half-life | ~3-21 days | <1 hour, can be extended via conjugation to PEG, nanoparticles, or VHH domains that bind serum albumin |
| Affinity range | Very high affinity constants from 10 pM-200 pM (median 66 pM)14 | Nanomolar to subnanomolar affinity constants; can be modulated depending on application needs11,15 |
Modalities of Antibody Engineering
Effective antibody engineering requires reliable, high-throughput, and cost-effective methods. In the conventional hybridoma method, B cells are isolated from immunized animals and fused to myeloma cells, which can expose cells to genetic drift, introducing potential stability and specificity problems. Scaling up manufacturing can also be slow and challenging.
To overcome these limitations, recombinant antibodies can be created in vitro without the need for animal immunization. This approach allows for the perpetual production of identical antibodies and enables genetic modifications and substitution of structural parts with human or other animals. This genetic flexibility also supports the development of bispecific antibodies, where a single molecule recognizes and crosslinks two different antigens. Moreover, the known DNA sequence of the antigen binding site reduces the risk of genetic drift and loss.
Developing recombinant antibodies by phage display bypasses the restrictions of immunization and can generate fully human antibodies via an in vitro selection process.16 First used over three decades ago, phage display enables the discovery of high-affinity antibodies by packaging an antibody gene inside a phage in a way that also expresses the antibody on the surface of the phage. Large libraries of unique phages are then exposed to the target, and bound phages are eluted, used for E. coli infection, and amplified to enrich for target antibody clones.16
scFvs, Fabs, VHH, and bispecific antibody fragments are commonly developed using phage display.17 The advantages of phage display engineering over hybridoma are multifold: it does not have to rely on the limitations of immunization and can use human gene repertoires.16 As a result, recombinant antibodies produced via phage display maintain high specificity and low immunogenicity, and they can be easily selected from display libraries because their immune specificity is fully recovered.11
Downstream Engineering and Applications of Monoclonal Antibodies and VHH
Once discovered and produced by phage display, VHH antibodies carry the advantage of simplified downstream engineering compared to mAbs. Their single domain structure simplifies affinity maturation and developability, and multiple VHH domains can be linked or fused for desired functionality like activation, clustering, blocking, and specificity to multiple targets. They are more easily amenable to bispecific or trispecific formats for applications like immuno-oncology. And their increased stability allows for better performance in harsher conditions allowing for not only greater opportunities in drug discovery, but also agricultural and industrial applications13.
Owing to their small size and deeper penetrance, VHH have powerful potential in cancer diagnostics and therapeutics. For molecular imaging, labeling with VHH may lead to faster biodistribution and renal clearance of the unbound portion, potentially minimizing radiation exposure. Other diagnostic applications include tumor identification, tumor stroma visualization, and immune infiltration monitoring.7 VHH can also be linked to fluorophores and radionuclides to enable site-specific derivatization.11
The biophysical properties of VHH make them amenable to implementation in cancer therapeutics, including targeting tumor antigens, enhancing immune checkpoint inhibitors, and blocking tumor angiogenesis.7 VHH can be engineered so that they can penetrate the blood-brain barrier, opening up possibilities in brain cancers and other neurological conditions.18
Furthermore, mAbs and antibody fragments are commonly used as reagents in the manufacture of drug substances. One notable application is immunoaffinity chromatography, where mAbs attached to a solid support are employed to purify drug substances. These ancillary applications benefit from the high stability in physical and performance characteristics.19
The Future of Antibody Engineering
The antibody landscape will continue to evolve, with VHH and other non-canonical antibodies playing a greater role in personalized medicine. As antibody engineering approaches continue to march ahead, research and clinical methods will expand beyond the limitations of conventional mAbs and ultimately offer more precisely tailored options to patients.
Unlock the Potential of Your Research
Whether you are looking to start a custom project tailored specifically to meet your needs, or purchase off-the-shelf antibodies from our rapidly growing catalog containing more than 9,500 products, our team is here to support your research and therapeutic goals. Explore our offerings and find the perfect antibody solutions for your applications. Contact us today to learn more and get started!
References
- Mullard A. Nat Rev Drug Discov. 2021;20(7):491-495.
- Crescioli S, et al. MAbs. 16(1):2297450.
- Hamers-Casterman C, et al. Nature. 1993;363(6428):446-448.
- Bannas P, et al. Front Immunol. 2017;8:1603.
- Berland L, et al. Biomolecules. 2021;11(5):637.
- De Genst E, et al. PNAS. 2006;103(12):4586-4591.
- Yang EY, Shah K. Front Oncol. 2020;10:1182.
- ClinicalTrials.gov. Accessed July 19, 2024.
- Bates A, et al. Antibodies (Basel). 2019;8(2):28.
- Jovčevska I, et al. BioDrugs. 2020;34(1):11-26.
- Gonzalez-Sapienza G, et al. Front Immunol. 2017;8:288027.
- Harmsen MM, et al. Appl Microbiol Biotechnol. 2007;77(1):13-22.
- Peltomaa R, et al. Anal Bioanal Chem. 2022;414(1):193-217.
- Landry JP, et al. J Immunol Methods. 2015;417:86-96.
- De Groof TWM, et al. Mol Cell Endocrinol. 2019;484:15-24.
- Roth KDR, et al. Front Cell Infect Microbiol. 2021;11:697876.
- Zhang Y. MAbs. 15(1):2213793.
- Tsitokana ME, et al. Int J Mol Sci. 2023;24(3):2632.
- FDA. Guidance for industry: monoclonal antibodies used as reagents in drug manufacturing. March 2001. Accessed July 19, 2024.